Question
A mixture containing 65.0 mole% acetone (Ac) and the balance acetic acid (AA) is separated in a continuous distillation column at 1 atm. A flowchart
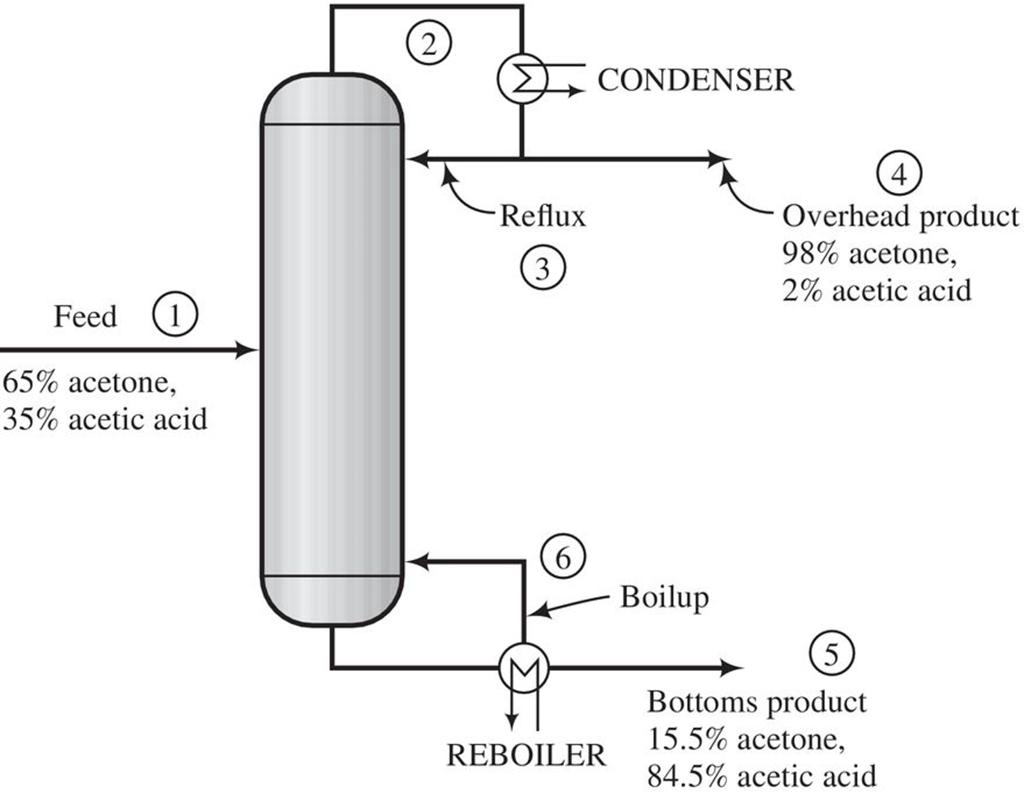
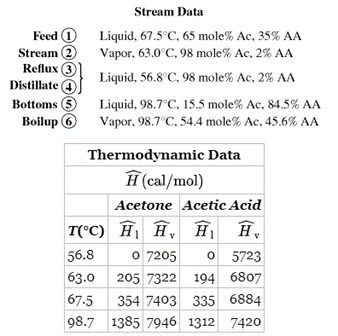
A mixture containing 65.0 mole% acetone (Ac) and the balance acetic acid (AA) is separated in a continuous distillation column at 1 atm. A flowchart for the operation is shown below. The stream from the top of the column is a vapor that passes though a condenser. The condensed liquid is divided into two equal streams: one is taken off as the overhead product (distillate) and the other (the reflux) is returned to the column. The stream from the bottom of the column is a liquid that is partially vaporized in a reboiler. The liquid stream emerging from the reboiler is taken off as the bottoms product, and the vapor is returned to the column as boilup. Negligible heat is lost from the column, so that the only places in the system where external heat transfer takes place are the condenser and the reboiler.
a. Taking 100 mol of feed as a basis, calculate the net heat requirement (cal) for the process. (You may neglect heats of mixing, although doing so for dissimilar liquids like acetone and acetic acid may introduce some error.)
b. For the same 100 mol of feed basis, calculate the required heat input to the reboiler and the required heat removal from the condenser.
c. Suppose that instead of the condensed liquid from the top of column being split into two equal streams to form the reflux and the overhead product, it is split into two streams, with the reflux being 3 times the overhead product. As before, take a basis of 100 mol of feed and determine the net heating requirements (cal) for the process and the heat removed in the condenser and added in the reboiler.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


