Answered step by step
Verified Expert Solution
Question
1 Approved Answer
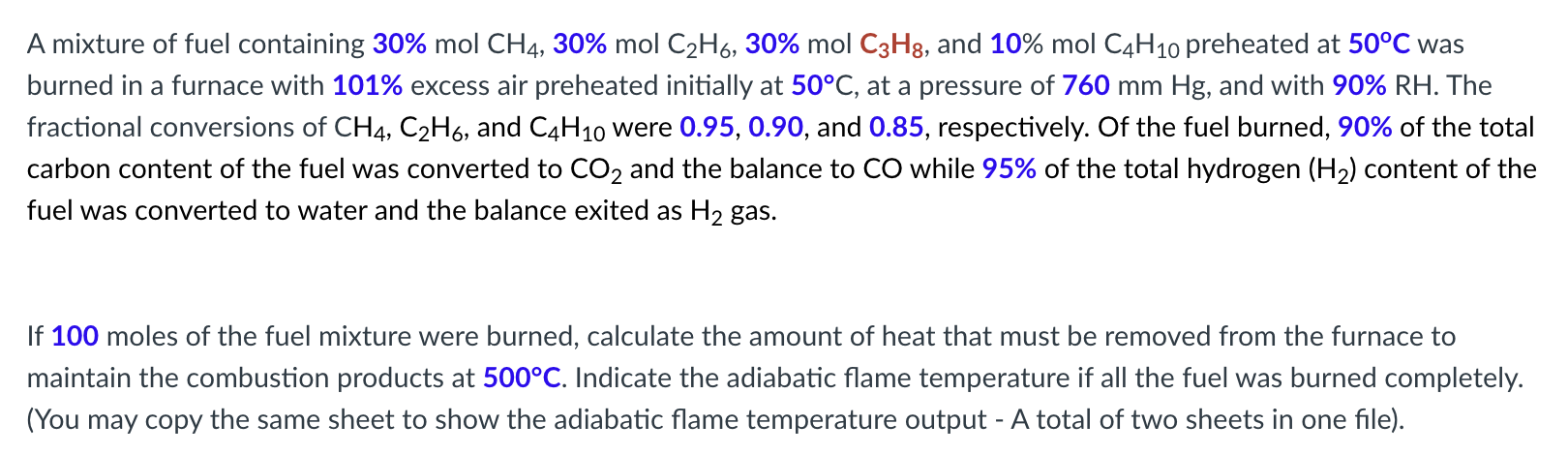
A mixture of fuel containing 3 0 % m o l C H 4 , 3 0 % m o l C 2 H 6
A mixture of fuel containing and mol preheated at was burned in a furnace with excess air preheated initially at at a pressure of and with The fractional conversions of and were and respectively. Of the fuel burned, of the total carbon content of the fuel was converted to and the balance to while of the total hydrogen content of the fuel was converted to water and the balance exited as gas.
If moles of the fuel mixture were burned, calculate the amount of heat that must be removed from the furnace to
maintain the combustion products at Indicate the adiabatic flame temperature if all the fuel was burned completely.
You may copy the same sheet to show the adiabatic flame temperature output A total of two sheets in one file
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


