Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a. Normality of the STD. Base b. Normality of the STD. Acid c. Wt of pure ASA in grams d. H of Aspirin e. %
a. Normality of the STD. Base
A 0.9550 g sample of ASA was dissolved in 40.1 mL of standard NaOH. The excess NaOH was neutralized using 18.1 ml of a standard HCI Note that 1.0 mL of the standard acid is equivalent to 0.0273 g sodium carbonate and 1,0 mL of the standard base is equivalent to 0.1005 g KHP. MW: KHP = 204 22 Na2CO3 = 106.0 ASA = 180.16 ASA + NaOH +H2O OH + NaOH 2 +CH COONa Na acetate Na Na Acetylsalicylate x'ss NaOH + HCI Na salicylate NaCl + H2O A 0.9550 g sample of ASA was dissolved in 40.1 mL of standard NaOH. The excess NaOH was neutralized using 18.1 ml of a standard HCI Note that 1.0 mL of the standard acid is equivalent to 0.0273 g sodium carbonate and 1,0 mL of the standard base is equivalent to 0.1005 g KHP. MW: KHP = 204 22 Na2CO3 = 106.0 ASA = 180.16 ASA + NaOH +H2O OH + NaOH 2 +CH COONa Na acetate Na Na Acetylsalicylate x'ss NaOH + HCI Na salicylate NaCl + H2O b. Normality of the STD. Acid
c. Wt of pure ASA in grams
d. H of Aspirin
e. % ASA in the sample 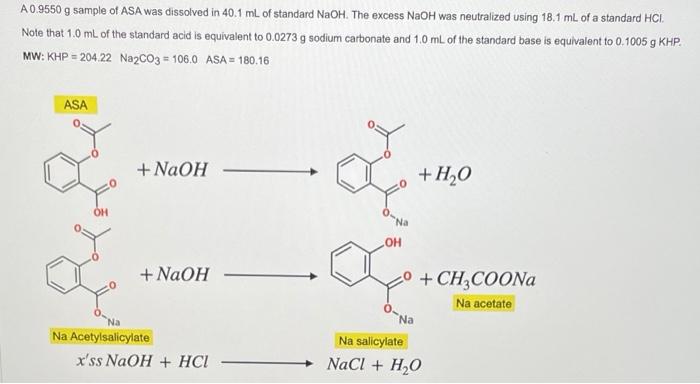

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


