Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A only. P6.38 If the reaction Fe2N(s)+3/2H2(g) 2Fe(s)+NH3(g) comes to equilibrium at a total pressure of 1 bar, analysis of the gas shows that at
A only. 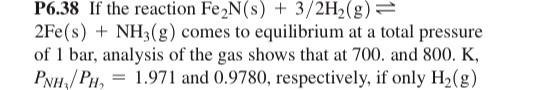

P6.38 If the reaction Fe2N(s)+3/2H2(g) 2Fe(s)+NH3(g) comes to equilibrium at a total pressure of 1 bar, analysis of the gas shows that at 700. and 800.K, PNH3/PH2=1.971 and 0.9780 , respectively, if only H2(g) was initially present in the gas phase and Fe2N(s) was in excess. a. Calculate KP at 700 . and 800.K. b. Calculate rS at 700.K and 800.K and rH, assuming that it is independent of temperature. c. Calculate rG for this reaction at 298.15K 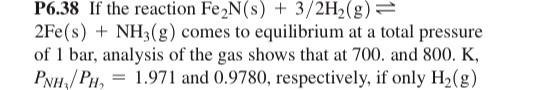

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


