Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A packed bed column removes CO2 from air by absorbing it into water (equilibrium diagram above). At a specific location in the column, the bulk
A packed bed column removes CO2 from air by absorbing it into water (equilibrium diagram above). At a specific location in the column, the bulk mole fractions of CH4 in the air and the water phases are 16 mol% and 2 mol%, respectively. The ky at this specific location is 0.04 kg mol/sm2, and kx is 0.12 kg mol/sm2. Calculate the y* and x* at this point. Calculate the Ky and Kx and obtain the fluxes in the vapor and liquid phase using the overall mass transfer coefficients.
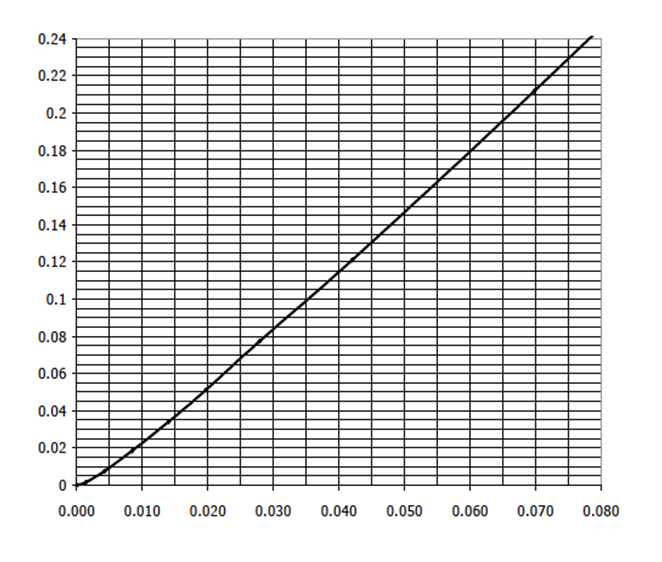
0.24 0.22 0.2 0.18 0.16 0.14 0.12 0.1 0.08 0.06 0.04 0.02 0 0.000 0.010 0.020 0.030 0.040 0.050 0.060 0.070 0.080
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


