Question
A packed column is used to absorb a toxic pollutant from a gas stream. From the data given below, calculate the height of packing and
A packed column is used to absorb a toxic pollutant from a gas stream. From the data given below, calculate the height of packing and column diameter. The unit operates at 50% of the flooding gas mass velocity, the actual liquid flow rate is 40% more than the minimum, and 95% of the pollutant is to be collected. Employ the generalized correlation provided in Figure 4 to estimate the column diameter. Gas mass flow rate = 3500 lb/h Pollutant concentration in inlet gas stream = 1.1 mol% Scrubbing liquid = pure water Packing type = 1-inch Raschig rings; packing factor F = 160 HOG of the column = 2.5 ft Henrys law constant m = 0.98 Density of gas (air) = 0.075 lb/ft3 Density of water = 62.4 lb/ft3 Viscosity of water = 1.8 cP 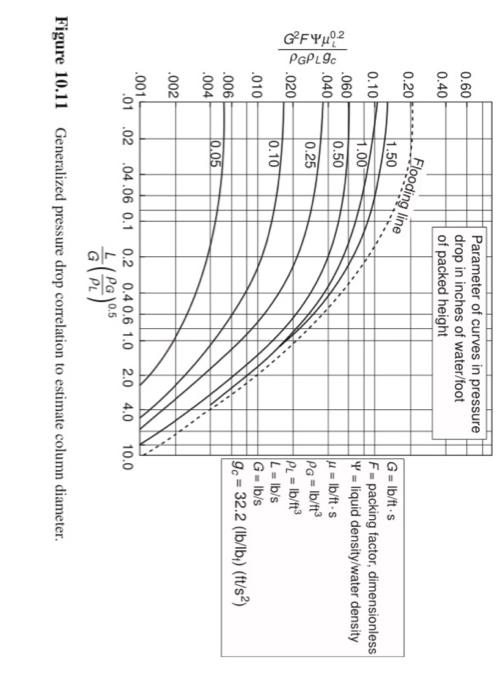
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


