Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A packed tower is to be designed to remove 85% of the ammonia(NH3) from a gaseous mixture of 9% ammonia and 91% air, by volume
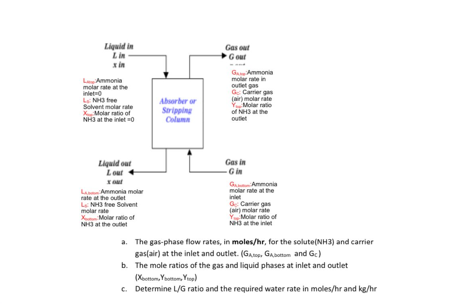
A packed tower is to be designed to remove 85% of the ammonia(NH3) from a gaseous mixture of 9% ammonia and 91% air, by volume . Molar flow rate of the inlet gaseous mixture is 100 mol/hr. The equilibrium and operating line for this water-ammonia are given below. X and Y values are molar rations in liquid and gaseous phase, respectively.
Liquid in Lin Xin Armonia molar att iniet Latre Solvent molar rate Molar ratio of NHI at the net Gescu Goat Gemmonia morate in outlet G Carreras (molar rate Y Morato of NH3 at the outlet Absorber or Stripping Liquidoar Gas in Lour Gin Ammonia Ammonia molar molare rate at the outlet niet NH43tree Solvent Cameras molarrate (molarrate Molar ratio of Y More of Not the outlet at the net a. The gas-phase flow rates, in moles/hr, for the solute(NH3) and carrier gas(air) at the inlet and outlet. (Glop Gbottom and Gc) b. The mole ratios of the gas and liquid phases at inlet and outlet Xoottom. Yorom. Yol c. Determine L/Gratio and the required water rate in moles/hr and kg/hr
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


