Answered step by step
Verified Expert Solution
Question
1 Approved Answer
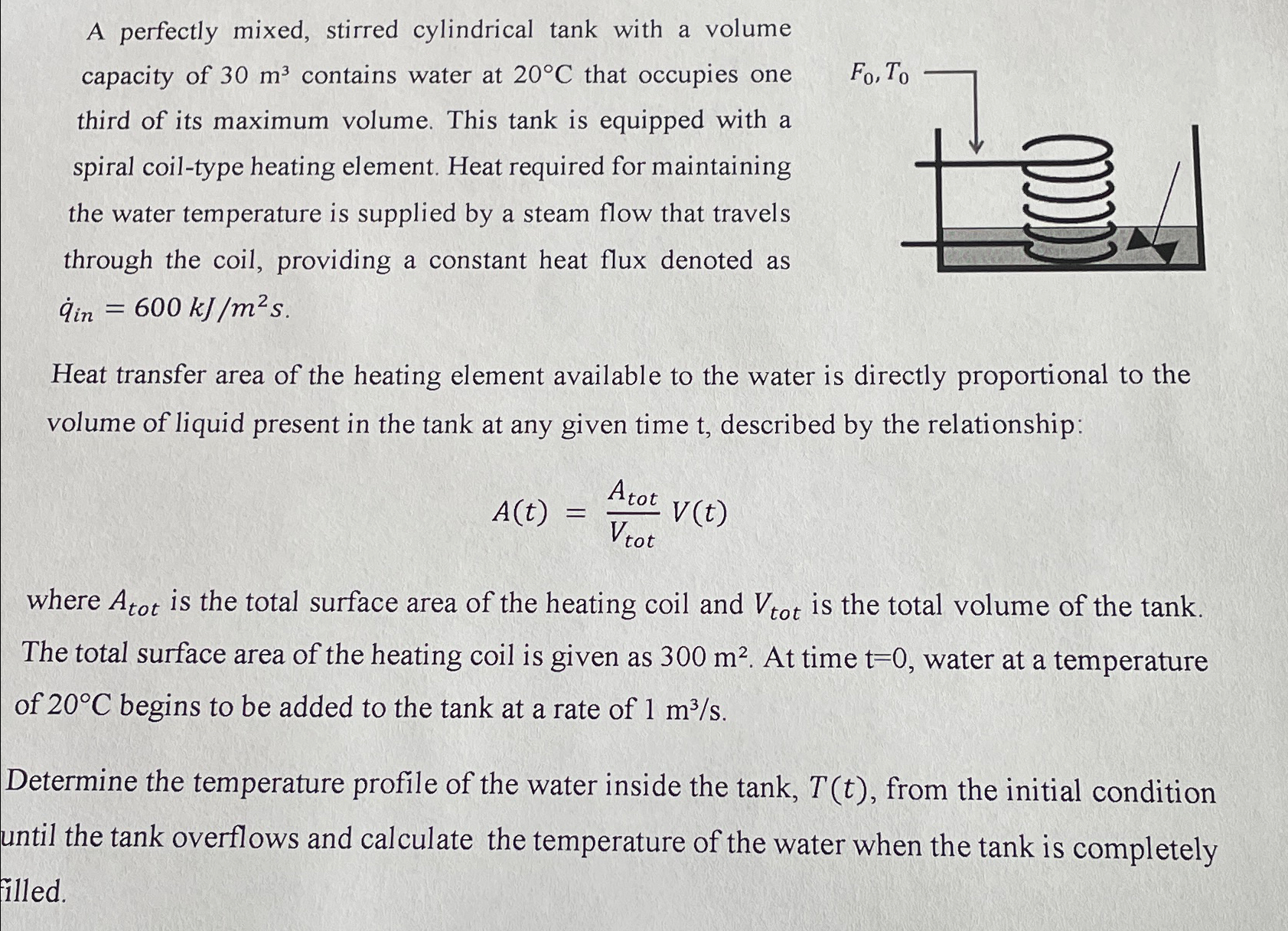
A perfectly mixed, stirred cylindrical tank with a volume capacity of 3 0 m 3 contains water at 2 0 C that occupies one third
A perfectly mixed, stirred cylindrical tank with a volume capacity of contains water at that occupies one third of its maximum volume. This tank is equipped with a spiral coiltype heating element. Heat required for maintaining the water temperature is supplied by a steam flow that travels through the coil, providing a constant heat flux denoted as
Heat transfer area of the heating element available to the water is directly proportional to the volume of liquid present in the tank at any given time described by the relationship:
where is the total surface area of the heating coil and is the total volume of the tank. The total surface area of the heating coil is given as At time water at a temperature of begins to be added to the tank at a rate of
Determine the temperature profile of the water inside the tank, from the initial condition until the tank overflows and calculate the temperature of the water when the tank is completely illed.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


