Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A piston-cylinder device that containing 35 kg of refrigerant R-134a initially has a volume of 0.58 m at 38C. The piston-cylinder device is then
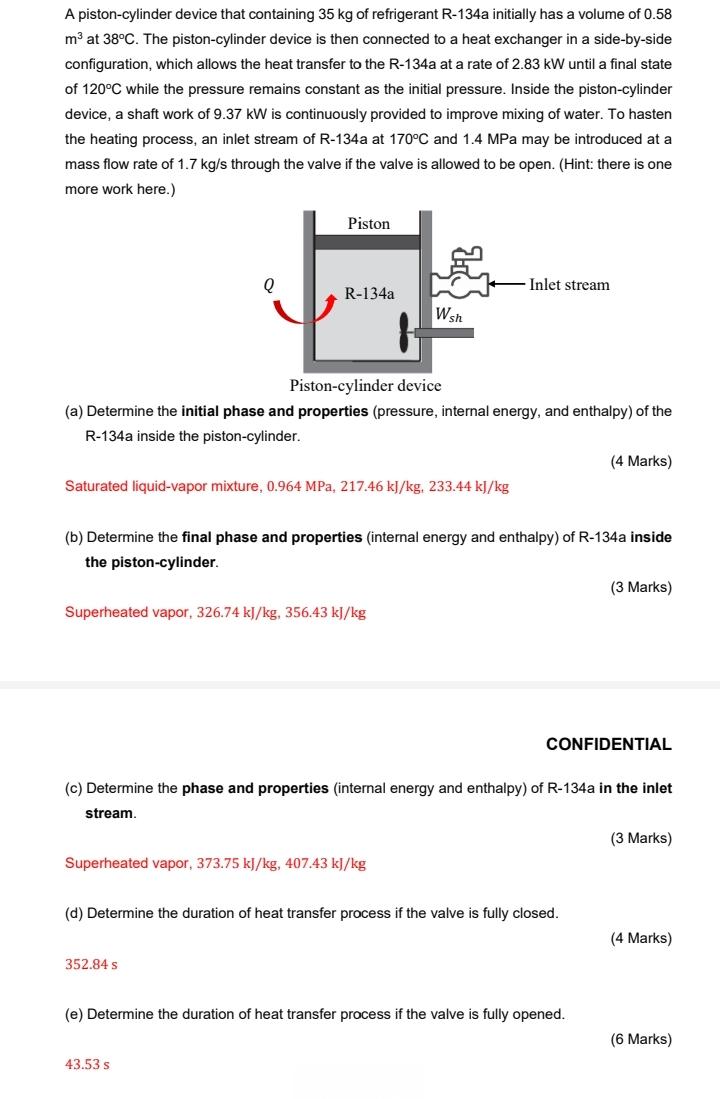
A piston-cylinder device that containing 35 kg of refrigerant R-134a initially has a volume of 0.58 m at 38C. The piston-cylinder device is then connected to a heat exchanger in a side-by-side configuration, which allows the heat transfer to the R-134a at a rate of 2.83 kW until a final state of 120C while the pressure remains constant as the initial pressure. Inside the piston-cylinder device, a shaft work of 9.37 kW is continuously provided to improve mixing of water. To hasten the heating process, an inlet stream of R-134a at 170C and 1.4 MPa may be introduced at a mass flow rate of 1.7 kg/s through the valve if the valve is allowed to be open. (Hint: there is one more work here.) Q Piston R-134a Piston-cylinder device (a) Determine the initial phase and properties (pressure, internal energy, and enthalpy) of the R-134a inside the piston-cylinder. Saturated liquid-vapor mixture, 0.964 MPa, 217.46 kJ/kg, 233.44 kJ/kg Superheated vapor, 326.74 kJ/kg, 356.43 kJ/kg Wsh 352.84 S (b) Determine the final phase and properties (internal energy and enthalpy) of R-134a inside the piston-cylinder. Superheated vapor, 373.75 kJ/kg, 407.43 kJ/kg Inlet stream 43.53 s (c) Determine the phase and properties (internal energy and enthalpy) of R-134a in the inlet stream. (d) Determine the duration of heat transfer process if the valve is fully closed. (4 Marks) CONFIDENTIAL (e) Determine the duration of heat transfer process if the valve is fully opened. (3 Marks) (3 Marks) (4 Marks) (6 Marks)
Step by Step Solution
★★★★★
3.44 Rating (176 Votes )
There are 3 Steps involved in it
Step: 1
m05kg klp 500 N Ap 00m Patm P 100 kPa x085 100x10 12 Patm klP 100x10 500 Ap 446x4ufy1 16537 085X1979...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


