Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A piston-cylinder device with a spring connected to the piston contains molecular nitrogen (N2) at an initial temperature of 350 K and has an

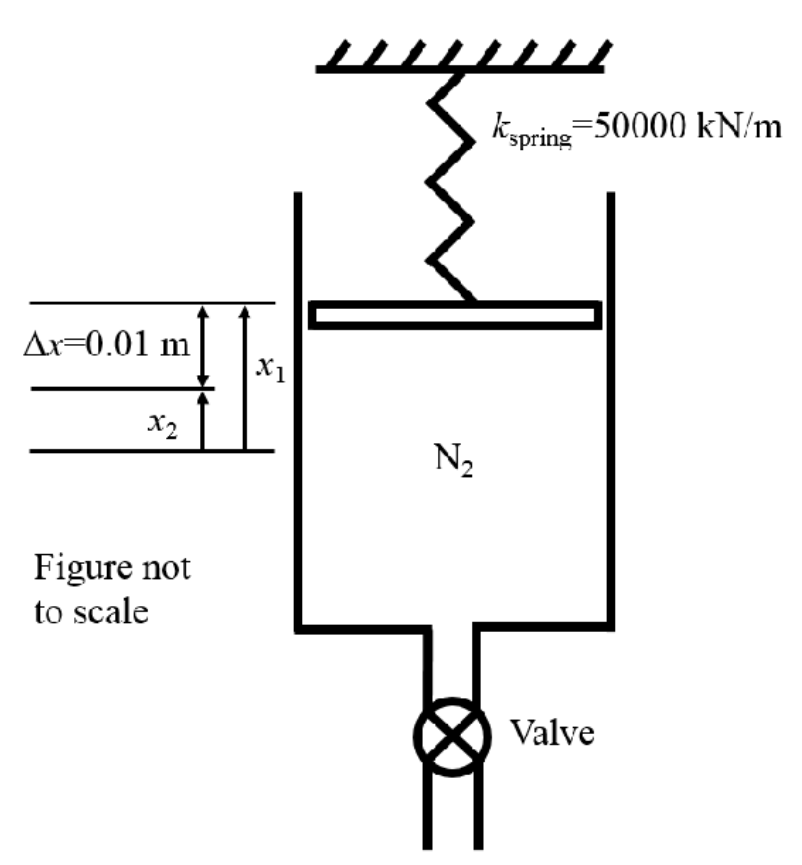
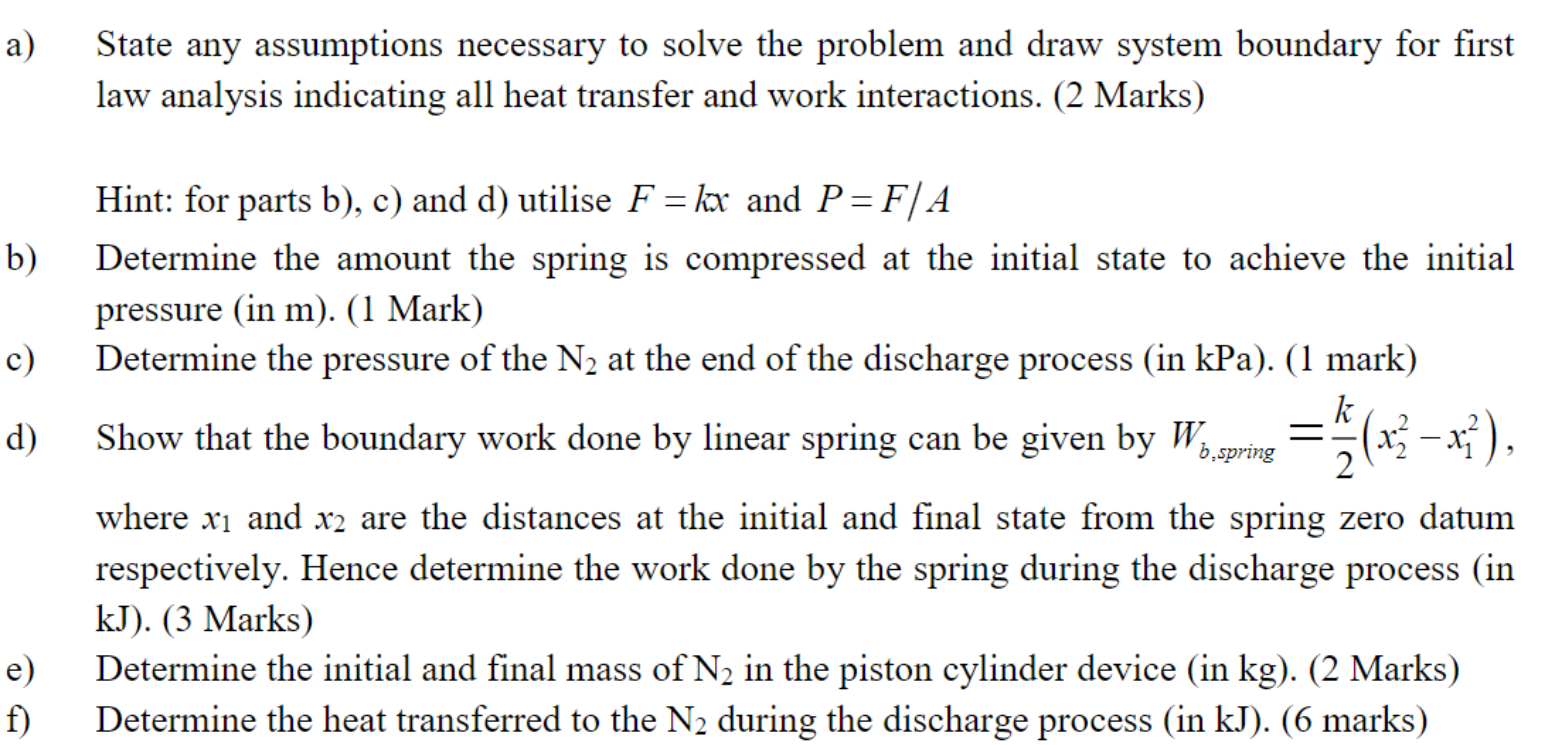
A piston-cylinder device with a spring connected to the piston contains molecular nitrogen (N2) at an initial temperature of 350 K and has an initial internal volume of 2.00 m. At the initial state, the linear spring with spring constant 50,000 kN/m applies a force to the piston such that the initial pressure of the N2 is 3000 kPa. The piston has a cross-sectional area of 1.5 m and the piston has negligible mass. At t=0 a discharge process is initiated by opening a valve such that N flows from the piston cylinder device until the piston is lowered by 0.01 m, at which point the discharge process is stopped by closing the valve. During the discharge process heat is transferred to the nitrogen in the piston-cylinder device such the process occurs in an isothermal manner. Assume that the N2 behaves as an ideal gas, if necessary the constant specific heat assumption may be employed. Ax=0.01 m m X2 X1 N Figure not to scale Kspring 50000 kN/m Valve a) b) c) d) e) f) State any assumptions necessary to solve the problem and draw system boundary for first law analysis indicating all heat transfer and work interactions. (2 Marks) Hint: for parts b), c) and d) utilise F = kx and P=F/A Determine the amount the spring is compressed at the initial state to achieve the initial pressure (in m). (1 Mark) Determine the pressure of the N at the end of the discharge process (in kPa). (1 mark) k Show that the boundary work done by linear spring can be given by W = 1/(x-x), b.spring 2 where x and x2 are the distances at the initial and final state from the spring zero datum respectively. Hence determine the work done by the spring during the discharge process (in kJ). (3 Marks) Determine the initial and final mass of N in the piston cylinder device (in kg). (2 Marks) Determine the heat transferred to the N during the discharge process (in kJ). (6 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


