Question
A plant for hydrogenating nitrobenzene (NB) consists of a tube reactor packed with catalyst of 1 inside diameter. 0.25 lbmol / h of a mixture:
A plant for hydrogenating nitrobenzene (NB) consists of a tube reactor packed with catalyst of 1 "inside diameter. 0.25 lbmol / h of a mixture: 2 mol% of NB and 98% mol hydrogen (H) are fed, at 150 ° C and 1 atmosphere. The wall temperature will be maintained at 150 ° C by means of a constant temperature bath. Hw = 20 BTU / h ft2 F. The overall reaction rate is:
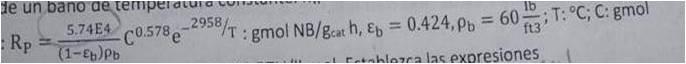
The heat of reaction AH = -274,000 BTU / lbmol. Set the expressions teeth to the solution of the One-Dimensional Model.
de un bano de temperatura 5.74E4 Rp = (1-Eb) Pb e-2958/T: gmol NB/gcat h, Eb = C0.578 e : gmol NB/gcat h, Ep = 0.424, pb = 60; ft3 Floara las expresiones ; T: C; C: gmol
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
hydrogenating nitis ben zene d 025 1b mul In ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



