Question
a. Plot the appropriate polarization curves for the following half cell reactions and determine the corrosion potential and corrosion rate (current density) assuming activation
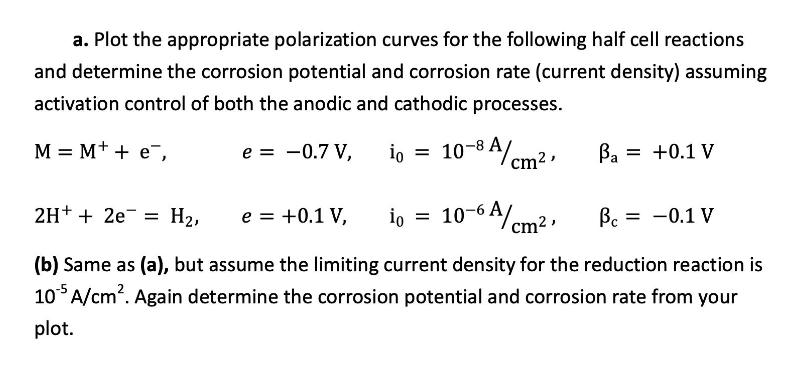
a. Plot the appropriate polarization curves for the following half cell reactions and determine the corrosion potential and corrosion rate (current density) assuming activation control of both the anodic and cathodic processes. 10-8A/cm, M = M+ + e, e = -0.7 V, io = io 10-6A/cm, = Ba = +0.1 V 2H+ + 2e H, e = +0.1 V, (b) Same as (a), but assume the limiting current density for the reduction reaction is 105 A/cm. Again determine the corrosion potential and corrosion rate from your plot. = -0.1 V
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Materials Science and Engineering An Introduction
Authors: William D. Callister Jr., David G. Rethwisch
8th edition
470419970, 978-0470419977
Students also viewed these Programming questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



