Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A power cycle absorbs 100 MW heat from a boiler at 800C and expels waste heat to cooling water at 25C. You can assume that
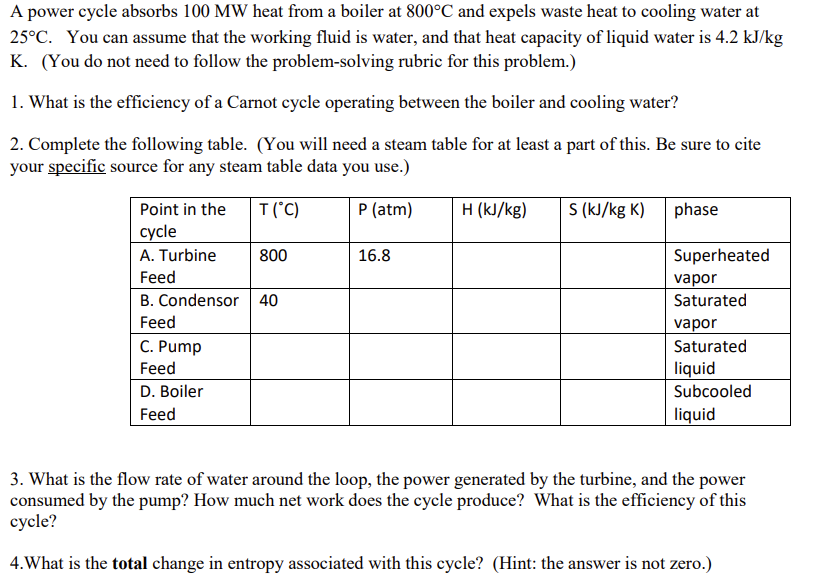
A power cycle absorbs 100 MW heat from a boiler at 800C and expels waste heat to cooling water at 25C. You can assume that the working fluid is water, and that heat capacity of liquid water is 4.2 kJ/kg K. (You do not need to follow the problem-solving rubric for this problem.) 1. What is the efficiency of a Carnot cycle operating between the boiler and cooling water? 2. Complete the following table. (You will need a steam table for at least a part of this. Be sure to cite your specific source for any steam table data you use.) Point in the T (C) P (atm) H (kJ/kg) S (kJ/kg K) phase cycle A. Turbine 800 16.8 Superheated Feed vapor B. Condensor 40 Saturated Feed vapor C. Pump Saturated Feed D. Boiler Feed liquid Subcooled liquid 3. What is the flow rate of water around the loop, the power generated by the turbine, and the power consumed by the pump? How much net work does the cycle produce? What is the efficiency of this cycle? 4. What is the total change in entropy associated with this cycle? (Hint: the answer is not zero.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


