Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A power plant needs to evaporate 1250.0 lb/h of water at 90.0F at 1 atm. Utility superheated steam at 1400F and 40 bar is available,
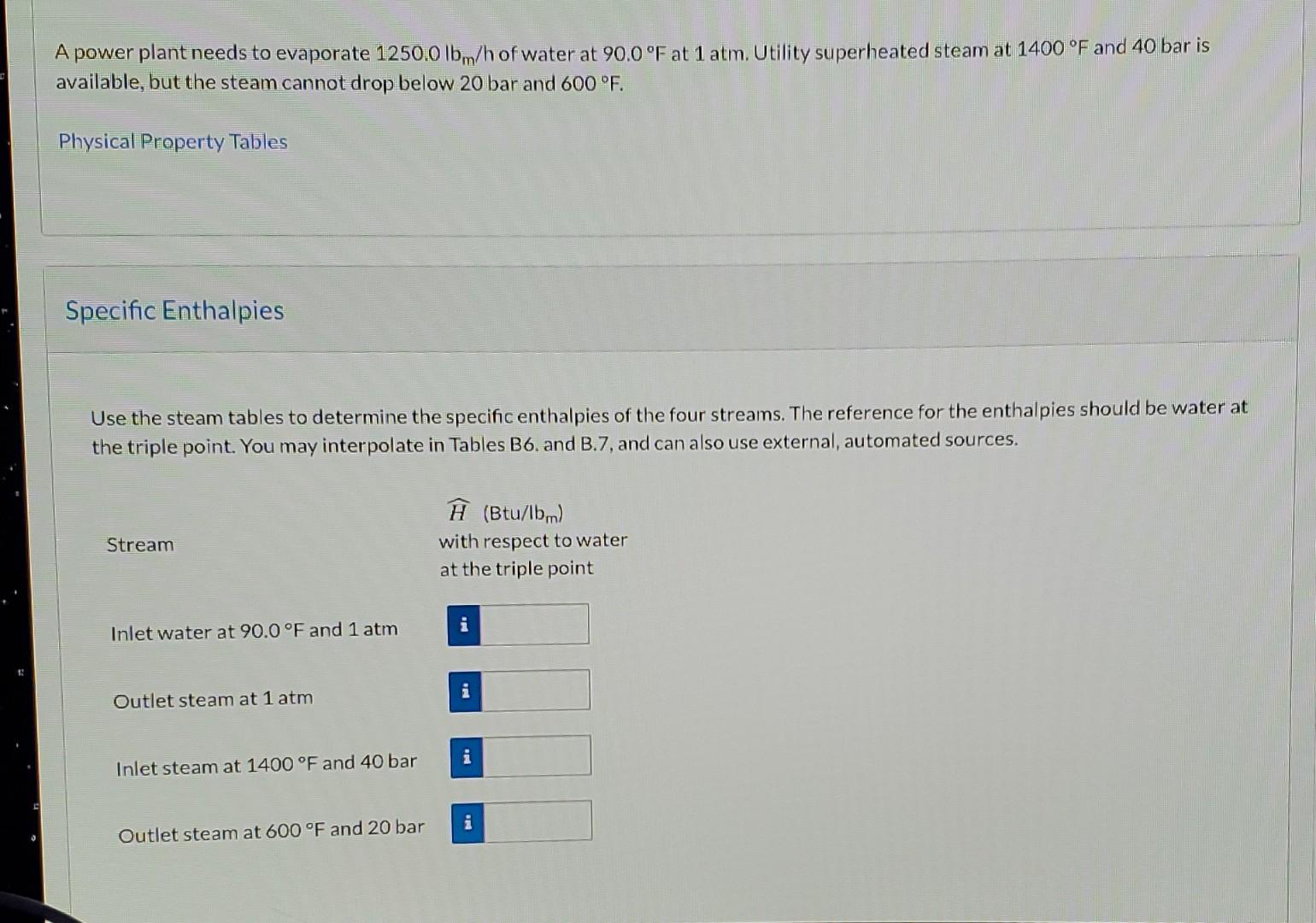
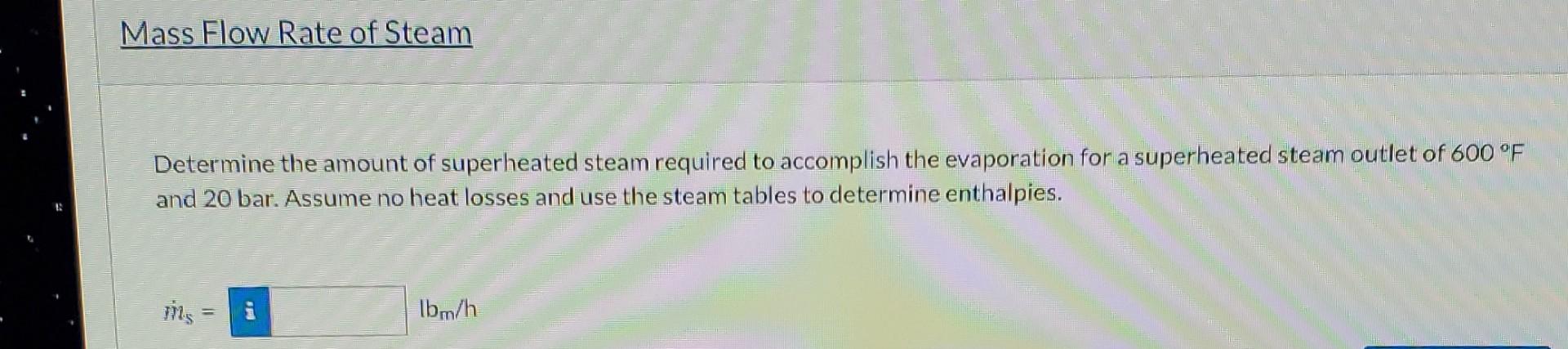
A power plant needs to evaporate 1250.0 lb/h of water at 90.0F at 1 atm. Utility superheated steam at 1400F and 40 bar is available, but the steam cannot drop below 20 bar and 600F. Physical Property Tables Specific Enthalpies Use the steam tables to determine the specific enthalpies of the four streams. The reference for the enthalpies should be water at the triple point. You may interpolate in Tables B6. and B.7, and can also use external, automated sources. Stream (Btu/lbm) with respect to water at the triple point i Inlet water at 90.0F and 1 atm i Outlet steam at 1 atm i Inlet steam at 1400F and 40 bar i Outlet steam at 600 F and 20 bar Mass Flow Rate of Steam Determine the amount of superheated steam required to accomplish the evaporation for a superheated steam outlet of 600 F and 20 bar. Assume no heat losses and use the steam tables to determine enthalpies. iN1s = Ibm/h
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


