Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A pressure cooker is used to cook food in a closed pot. By heating the contents of a pressure cooker at constant volume, the
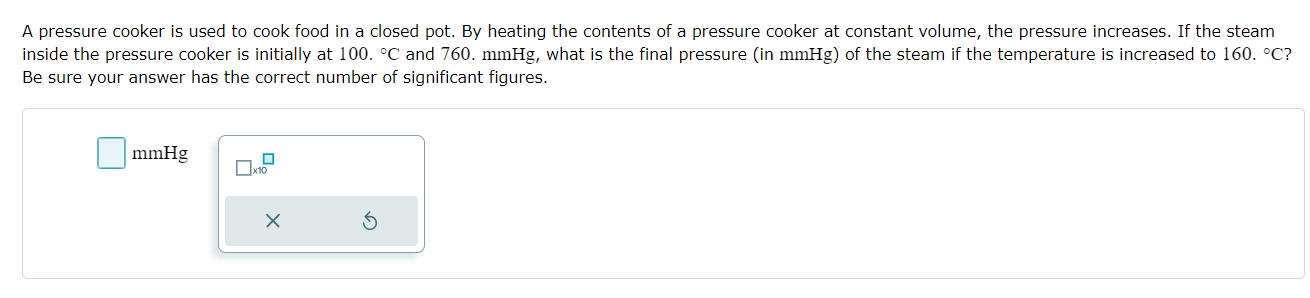
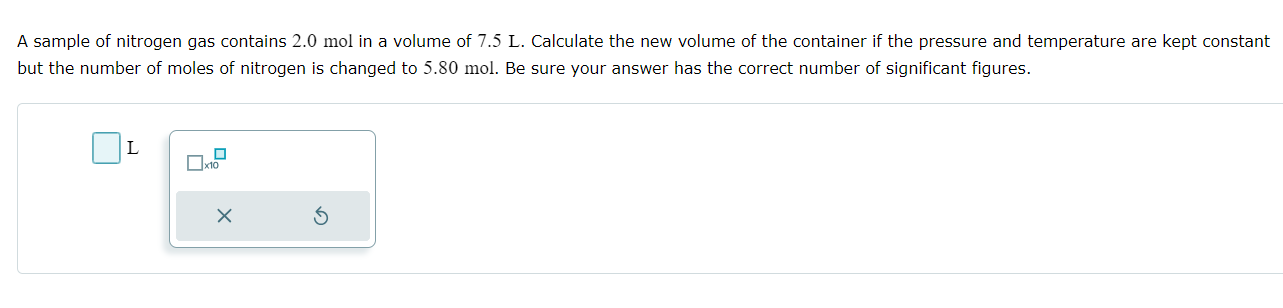
A pressure cooker is used to cook food in a closed pot. By heating the contents of a pressure cooker at constant volume, the pressure increases. If the steam inside the pressure cooker is initially at 100. C and 760. mmHg, what is the final pressure (in mmHg) of the steam if the temperature is increased to 160. C? Be sure your answer has the correct number of significant figures. mmHg x10 A sample of nitrogen gas contains 2.0 mol in a volume of 7.5 L. Calculate the new volume of the container if the pressure and temperature are kept constant but the number of moles of nitrogen is changed to 5.80 mol. Be sure your answer has the correct number of significant figures. L x10
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


