Question
A process stream consisting of solvent A with a flow of 3500 kg / h must be evaporated at a pressure of 5 bar so
A process stream consisting of solvent A with a flow of 3500 kg / h must be evaporated at a pressure of 5 bar so that a saturated steam is obtained. The process stream maintains the temperature at 20 ° C. Determine the heating effect which must be added to the process.
Other information:
cp (liquid) = 2.5 kJ kg-1 K-1
Steam generation heat at the boiling point of the solvent 5 bar: 900 kJ / kg
For substance A, the following parameters apply to Antoine's equation:
A = 15.9008 B = 2788.51 C = -52.36
Antoine's equation:
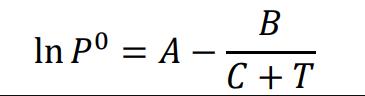
NOTE! The vapor pressure is given in mmHg (1 bar = 760 mmHg), the temperature in K
In P = A C +T
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
The heating ef...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Vector Mechanics for Engineers Statics and Dynamics
Authors: Ferdinand Beer, E. Russell Johnston, Jr., Elliot Eisenberg, William Clausen, David Mazurek, Phillip Cornwell
8th Edition
73212229, 978-0073212227
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



