Answered step by step
Verified Expert Solution
Question
1 Approved Answer
??? (a) Propose a stereoselective synthesis of chiral secondary alcohol 10 starting from Weinreb amide 9 [note: at least two steps are required and you
??? 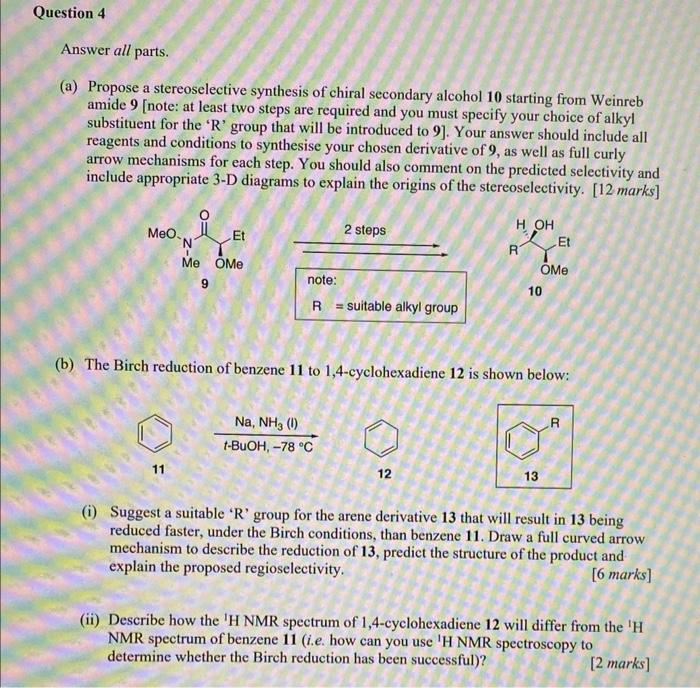
(a) Propose a stereoselective synthesis of chiral secondary alcohol 10 starting from Weinreb amide 9 [note: at least two steps are required and you must specify your choice of alkyl substituent for the ' R ' group that will be introduced to 9]. Your answer should include all reagents and conditions to synthesise your chosen derivative of 9, as well as full curly arrow mechanisms for each step. You should also comment on the predicted selectivity and include appropriate 3-D diagrams to explain the origins of the stereoselectivity. [12 marks] (b) The Birch reduction of benzene 11 to 1,4 -cyclohexadiene 12 is shown below: tBuOH,78CNa,NH3(1) 11 12 (i) Suggest a suitable ' R ' group for the arene derivative 13 that will result in 13 being reduced faster, under the Birch conditions, than benzene 11. Draw a full curved arrow mechanism to describe the reduction of 13, predict the structure of the product and explain the proposed regioselectivity. [6 marks] (ii) Describe how the ' H NMR spectrum of 1,4-cyclohexadiene 12 will differ from the ' H NMR spectrum of benzene 11 (i.e. how can you use ' H NMR spectroscopy to determine whether the Birch reduction has been successful)? [2 marks] 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


