Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A proton transfer reaction can occur when an aldehyde is placed in strong base, such as an alkoxide ion, producing an alcohol and a charged
A proton transfer reaction can occur when an aldehyde is placed in strong base, such as an alkoxide ion, producing an alcohol and a charged conjugate base that is resonance stabilized. In the left box, draw the curved arrows for the proton transfer. In the middle and right boxes, draw the two structures for the resonance-stabilized product as noted in the box-specific directions. Be sure to include all lone pairs and nonzero formal charges. 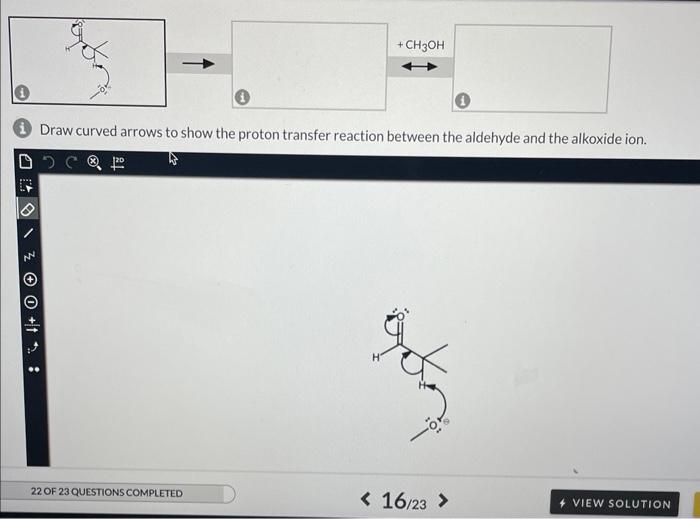
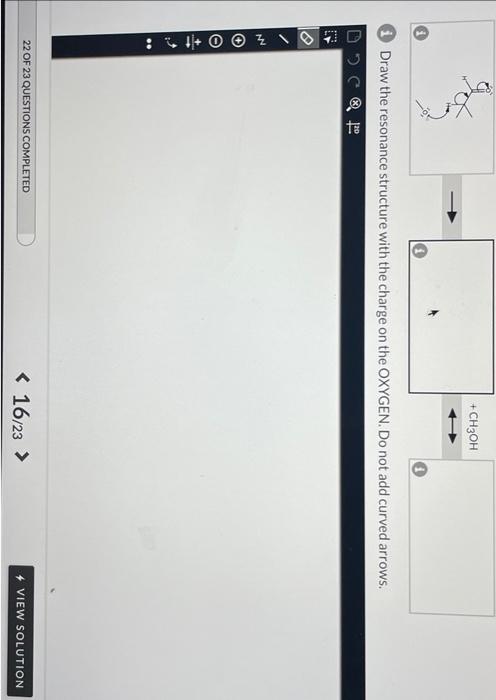
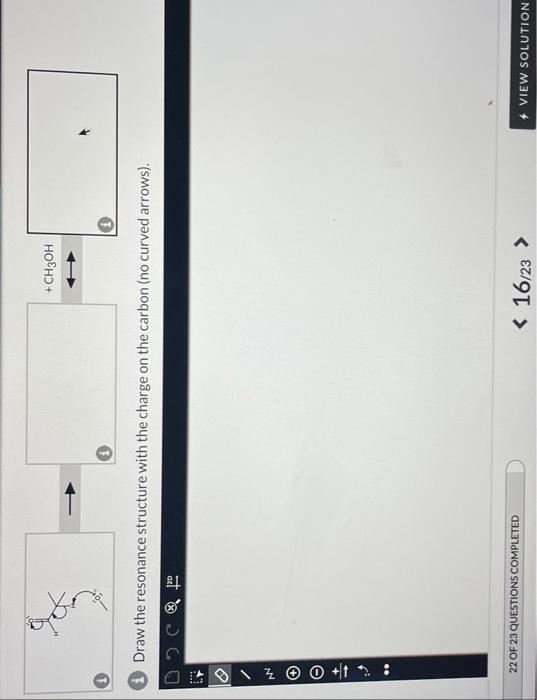
(i) Draw curved arrows to show the proton transfer reaction between the aldehyde and the alkoxide ion. (1) Draw the resonance structure with the charge on the OXYGEN. Do not add curved arrows. i) Draw the resonance structure with the charge on the carbon (no curved arrows) 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


