Answered step by step
Verified Expert Solution
Question
1 Approved Answer
. A proton, which is the nucleus of a hydrogen atom, can be modeled as a sphere with a diameter of 2.4 fm and a
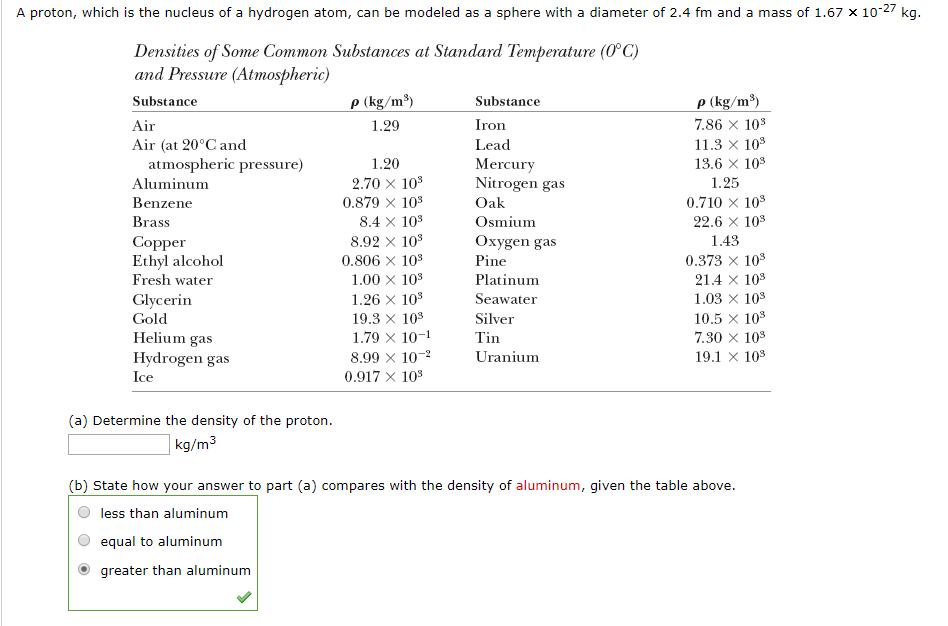 .
. A proton, which is the nucleus of a hydrogen atom, can be modeled as a sphere with a diameter of 2.4 fm and a mass of 1.67 x 10-27 kg. Densities of Some Common Substances at Standard Temperature (0C) and Pressure (Atmospheric) Substance Air Air (at 20C and atmospheric pressure) Aluminum Benzene Brass Copper Ethyl alcohol Fresh water Glycerin Gold Helium gas Hydrogen gas Ice (a) Determine the density of the proton. kg/m p (kg/m) 1.29 1.20 2.70 10 0.879 10 8.4 X 10 8.92 x 10 0.806 10 1.00 10 1.26 X 10 19.3 X 10 1.79 X 10-1 8.99 10- 0.917 x 10 Substance Iron Lead Mercury Nitrogen gas Oak Osmium Oxygen gas Pine Platinum Seawater Silver Tin Uranium P (kg/m) 7.86 x 10 11.3 x 10 13.6 10 1.25 0.710 10 22.6 10 1.43 0.373 x 108 21.4 x 10 1.03 10 10.5 10 7.30 X 10 19.1 x 10 (b) State how your answer to part (a) compares with the density of aluminum, given the table above. less than aluminum equal to aluminum greater than aluminum.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Density of a Proton and Comparison with Aluminum a Density of the Proton The densi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


