Question: A pulse oximeter operates by using light and a photocell to measure oxygen saturation in arterial blood. The transmission of light energy as it passes
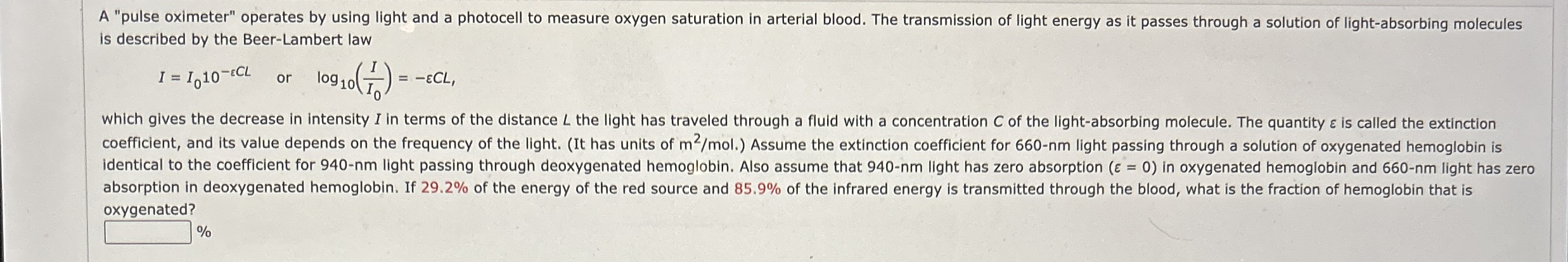
A "pulse oximeter" operates by using light and a photocell to measure oxygen saturation in arterial blood. The transmission of light energy as it passes through a solution of lightabsorbing molecules is described by the BeerLambert law
which gives the decrease in intensity I in terms of the distance the light has traveled through a fluid with a concentration of the lightabsorbing molecule. The quantity is called the extinction coefficient, and its value depends on the frequency of the light. It has units of Assume the extinction coefficient for light passing through a solution of oxygenated hemoglobin is identical to the coefficient for light passing through deoxygenated hemoglobin. Also assume that light has zero absorption in oxygenated hemoglobin and light has zero absorption in deoxygenated hemoglobin. If of the energy of the red source and of the infrared energy is transmitted through the blood, what is the fraction of hemoglobin that is oxygenated?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


