Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A quantum mechanics problem Schrdinger's equation in the absence of a potential is 2m22=E where is Planck's constant divided by 2,m is the mass, E
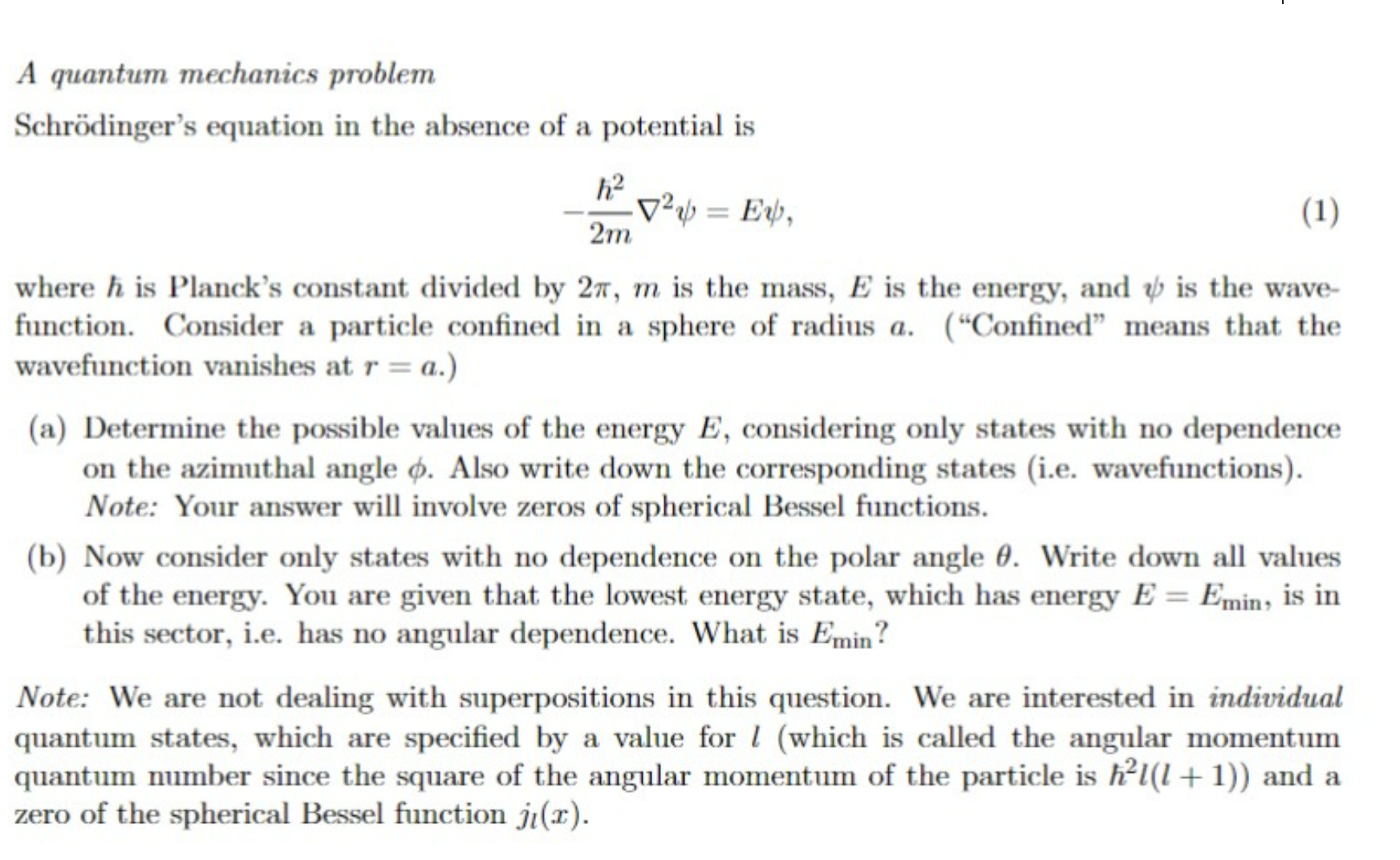 A quantum mechanics problem Schrdinger's equation in the absence of a potential is 2m22=E where is Planck's constant divided by 2,m is the mass, E is the energy, and is the wavefunction. Consider a particle confined in a sphere of radius a. ("Confined" means that the wavefunction vanishes at r=a.) (a) Determine the possible values of the energy E, considering only states with no dependence on the azimuthal angle . Also write down the corresponding states (i.e. wavefunctions). Note: Your answer will involve zeros of spherical Bessel functions. (b) Now consider only states with no dependence on the polar angle . Write down all values of the energy. You are given that the lowest energy state, which has energy E=Emin, is in this sector, i.e. has no angular dependence. What is Emin ? Note: We are not dealing with superpositions in this question. We are interested in individual quantum states, which are specified by a value for l (which is called the angular momentum quantum number since the square of the angular momentum of the particle is 2l(l+1)) and a zero of the spherical Bessel function jl(x)
A quantum mechanics problem Schrdinger's equation in the absence of a potential is 2m22=E where is Planck's constant divided by 2,m is the mass, E is the energy, and is the wavefunction. Consider a particle confined in a sphere of radius a. ("Confined" means that the wavefunction vanishes at r=a.) (a) Determine the possible values of the energy E, considering only states with no dependence on the azimuthal angle . Also write down the corresponding states (i.e. wavefunctions). Note: Your answer will involve zeros of spherical Bessel functions. (b) Now consider only states with no dependence on the polar angle . Write down all values of the energy. You are given that the lowest energy state, which has energy E=Emin, is in this sector, i.e. has no angular dependence. What is Emin ? Note: We are not dealing with superpositions in this question. We are interested in individual quantum states, which are specified by a value for l (which is called the angular momentum quantum number since the square of the angular momentum of the particle is 2l(l+1)) and a zero of the spherical Bessel function jl(x) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


