Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A reaction takes place in two CSTR reactors as shown in the diagram (parallel). A -> B -r(A) = kC(A) if k = 0.314 min^(-1),
A reaction takes place in two CSTR reactors as shown in the diagram (parallel). 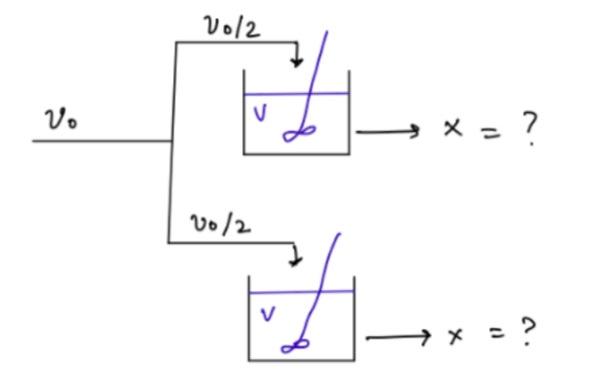
V012 V. X = ? 10/2 Ph V + x = A -> B -r(A) = kC(A)
if k = 0.314 min^(-1), V = 2000 (dm^3) for both CSTR reactors & v(0) = (dm^3/min)
a) Calculate the conversion (X(parallel))
b) Determine which case would yield a higher conversion rate, X(series) = 0.85 or X(parallel)

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


