Question
A rigid tank as part of a rocket engine contains 2 kg of Oxygen gas at 250 H and 1 MPa. 0.1 kmol of
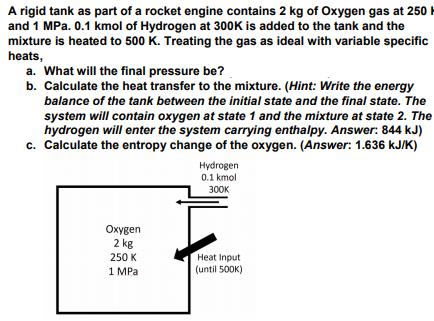
A rigid tank as part of a rocket engine contains 2 kg of Oxygen gas at 250 H and 1 MPa. 0.1 kmol of Hydrogen at 300K is added to the tank and the mixture is heated to 500 K. Treating the gas as ideal with variable specific heats, a. What will the final pressure be? b. Calculate the heat transfer to the mixture. (Hint: Write the energy balance of the tank between the initial state and the final state. The system will contain oxygen at state 1 and the mixture at state 2. The hydrogen will enter the system carrying enthalpy. Answer: 844 kJ) c. Calculate the entropy change of the oxygen. (Answer: 1.636 KJIK) Hydrogen 0.1 kmol 30 en 2 kg Heat Input 250 K 1 MPa (until 500K)
Step by Step Solution
3.33 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantitative Analysis For Management
Authors: Barry Render, Ralph M. Stair, Michael E. Hanna
11th Edition
9780132997621, 132149117, 132997622, 978-0132149112
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



