Answered step by step
Verified Expert Solution
Question
1 Approved Answer
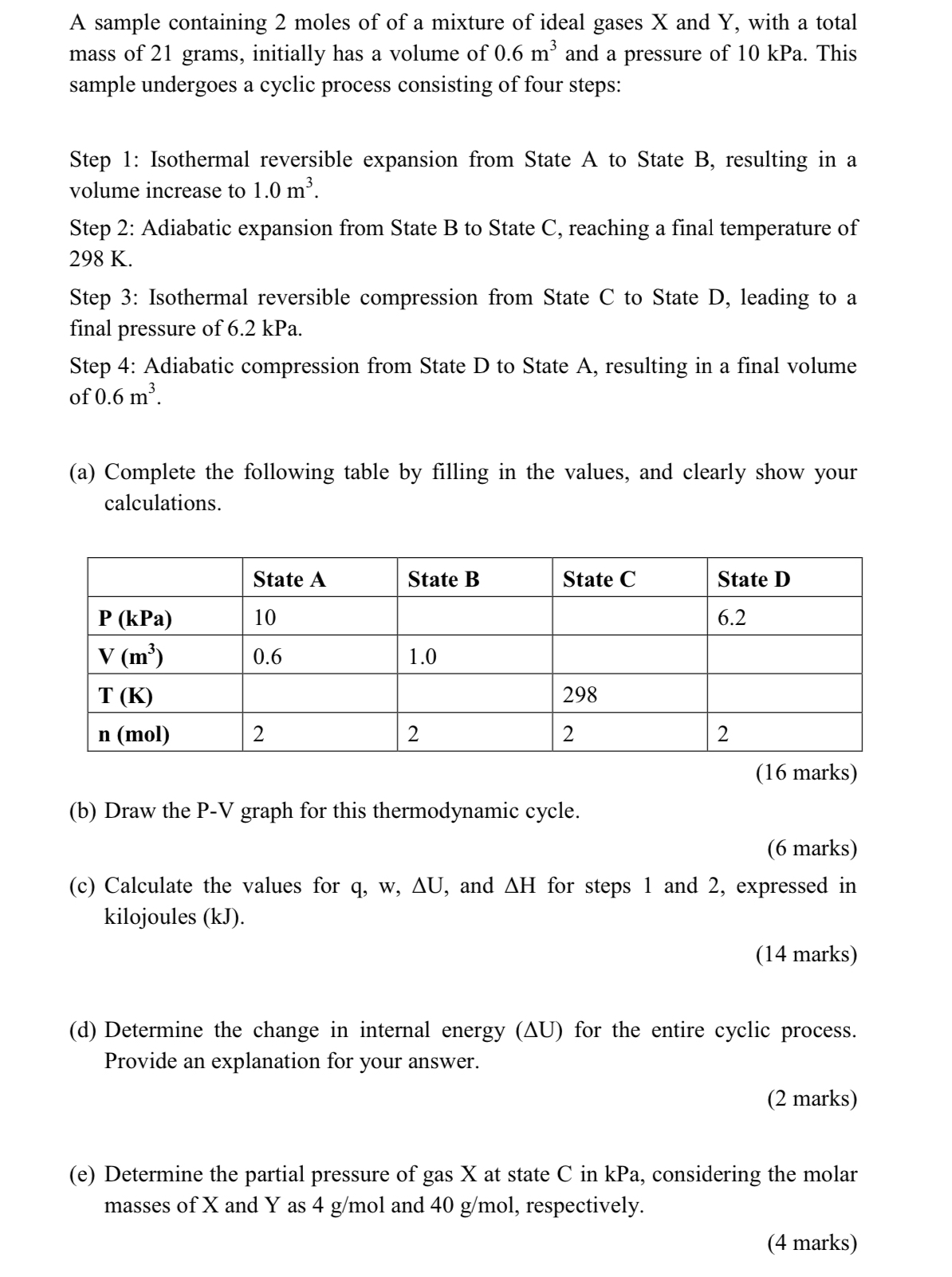
A sample containing 2 moles of of a mixture of ideal gases x and Y , with a total mass of 2 1 grams, initially
A sample containing moles of of a mixture of ideal gases and with a total mass of grams, initially has a volume of and a pressure of kPa. This sample undergoes a cyclic process consisting of four steps:
Step : Isothermal reversible expansion from State A to State B resulting in a volume increase to
Step : Adiabatic expansion from State B to State C reaching a final temperature of
Step : Isothermal reversible compression from State C to State D leading to a final pressure of kPa.
Step : Adiabatic compression from State D to State A resulting in a final volume of
a Complete the following table by filling in the values, and clearly show your calculations.
tableState AState BState CState D
marks
b Draw the PV graph for this thermodynamic cycle.
marks
c Calculate the values for and for steps and expressed in kilojoules
marks
d Determine the change in internal energy for the entire cyclic process. Provide an explanation for your answer.
marks
e Determine the partial pressure of gas at state in kPa, considering the molar masses of and as and respectively.
marks
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


