Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A series of N +1 large vats of water have temperatures To, T1, T2,..., TN (with T > Tn+1). A small body with heat
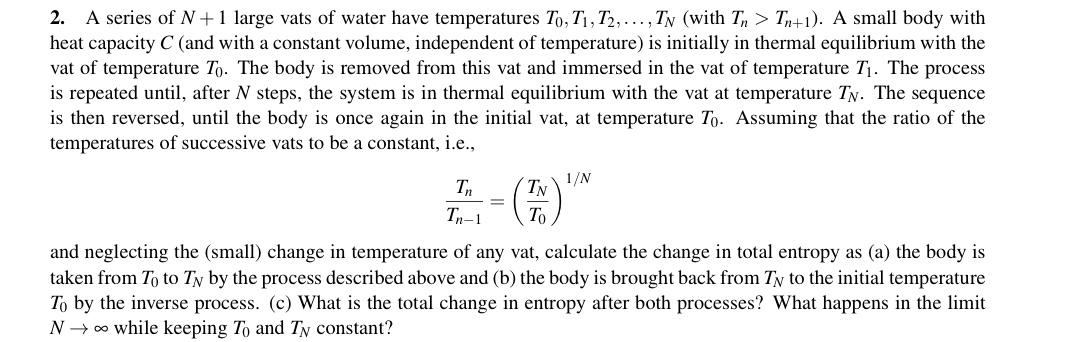
A series of N +1 large vats of water have temperatures To, T1, T2,..., TN (with T > Tn+1). A small body with heat capacity C (and with a constant volume, independent of temperature) is initially in thermal equilibrium with the vat of temperature To. The body is removed from this vat and immersed in the vat of temperature Tj. The process is repeated until, after N steps, the system is in thermal equilibrium with the vat at temperature TN. The sequence is then reversed, until the body is once again in the initial vat, at temperature To. Assuming that the ratio of the temperatures of successive vats to be a constant, i.e., 2. Tn 1/N Tn-1 TN To and neglecting the (small) change in temperature of any vat, calculate the change in total entropy as (a) the body is taken from To to TN by the process described above and (b) the body is brought back from TN to the initial temperature To by the inverse process. (c) What is the total change in entropy after both processes? What happens in the limit N o while keeping To and TN constant?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


