Question
A set of approximate bond energies could be obtained by considering the energy required to atomize a specific set of molecules. For example, combined results
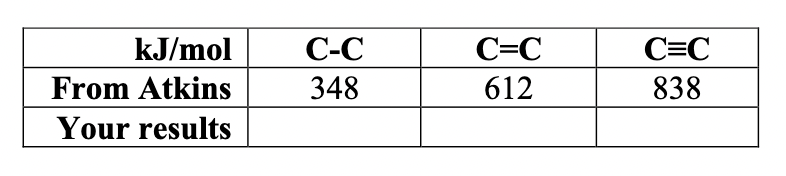
A set of approximate bond energies could be obtained by considering the energy required to atomize a specific set of molecules. For example, combined results for e(C-H) and e(O-H) (e = epsilon) (obtained from methane and water, respectively) to arrive at an approximate bond energy for the carbon-oxygen bond (e(C-O)) in methanol. (a) Use this same approach to find the bond energies in C-C using ethane, C=C using ethylene, and CC using acetylene (b) What are the bond energies in kJ/mol for the following: C-C, C=C and CC. Comment on results based on organic chemistry knowledge
H2O (g) --> 2H (g) + O (g) deltaH(rxn) = 926.93kJ/mol --> e(O-H) = 463 kJ/mol
CH4 (g) --> C (g) + 4H (g) deltaH(rxn) = 4e(C-H) = 1663.35 kJ/mol
OR deltaH(rxn) = [deltaH(formation)(C, g) + 4deltaH(formation)(H, g)] - deltaH(formation)(CH4, g) = 1663.35 kJ/mol
so, e(C-H) = deltaH(rxn)/4 = 415.8 kJ/mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


