Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A single effect evaporator has a thermal load, Qe of 2355 kJ/s and the heating steam temperature is 115 C. The seawater temperature, Tew,
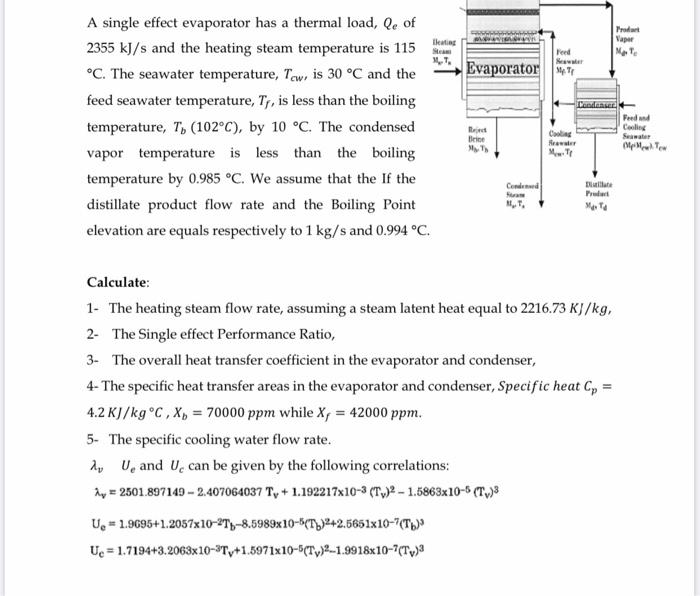
A single effect evaporator has a thermal load, Qe of 2355 kJ/s and the heating steam temperature is 115 C. The seawater temperature, Tew, is 30 C and the feed seawater temperature, Tr, is less than the boiling temperature, T, (102C), by 10 C. The condensed vapor temperature is less than the boiling temperature by 0.985 C. We assume that the If the distillate product flow rate and the Boiling Point elevation are equals respectively to 1 kg/s and 0.994 C. lleating Steam Evaporator U=1.9695+1.2057x10-2T-8.5989x10-5(T)2+2.5651x10-7(Th) Ue = 1.7194+3.2063x10-Ty+1.5971x10-5(Ty)-1.9918x10-7(Tv) Reject Brine My Th Condensed fram MT, Feed Seawater Cooling Seawater Condenser Distillate Product Max Ta Calculate: 1- The heating steam flow rate, assuming a steam latent heat equal to 2216.73 KJ/kg, 2- The Single effect Performance Ratio, 3- The overall heat transfer coefficient in the evaporator and condenser, 4- The specific heat transfer areas in the evaporator and condenser, Specific heat C = 4.2 KJ/kg C, X = 70000 ppm while X, = 42000 ppm. 5- The specific cooling water flow rate. AU and Ue can be given by the following correlations: Ay = 2501.897149-2.407064037 Ty+1.192217x10-3 (T)2-1.5863x10-5 (T,)3 Product Vapor Ma Te Feed and Cooling Seawater MeMe Tew
Step by Step Solution
★★★★★
3.30 Rating (171 Votes )
There are 3 Steps involved in it
Step: 1
ANSWER Given Thermal load Qe 2355 kJs Heating steam temperature T1 115C Seawater temperature Tew 30C Feed seawater temperature T2 92C Condensed vapor temperature T3 101015C Distillate product flow rat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


