Answered step by step
Verified Expert Solution
Question
1 Approved Answer
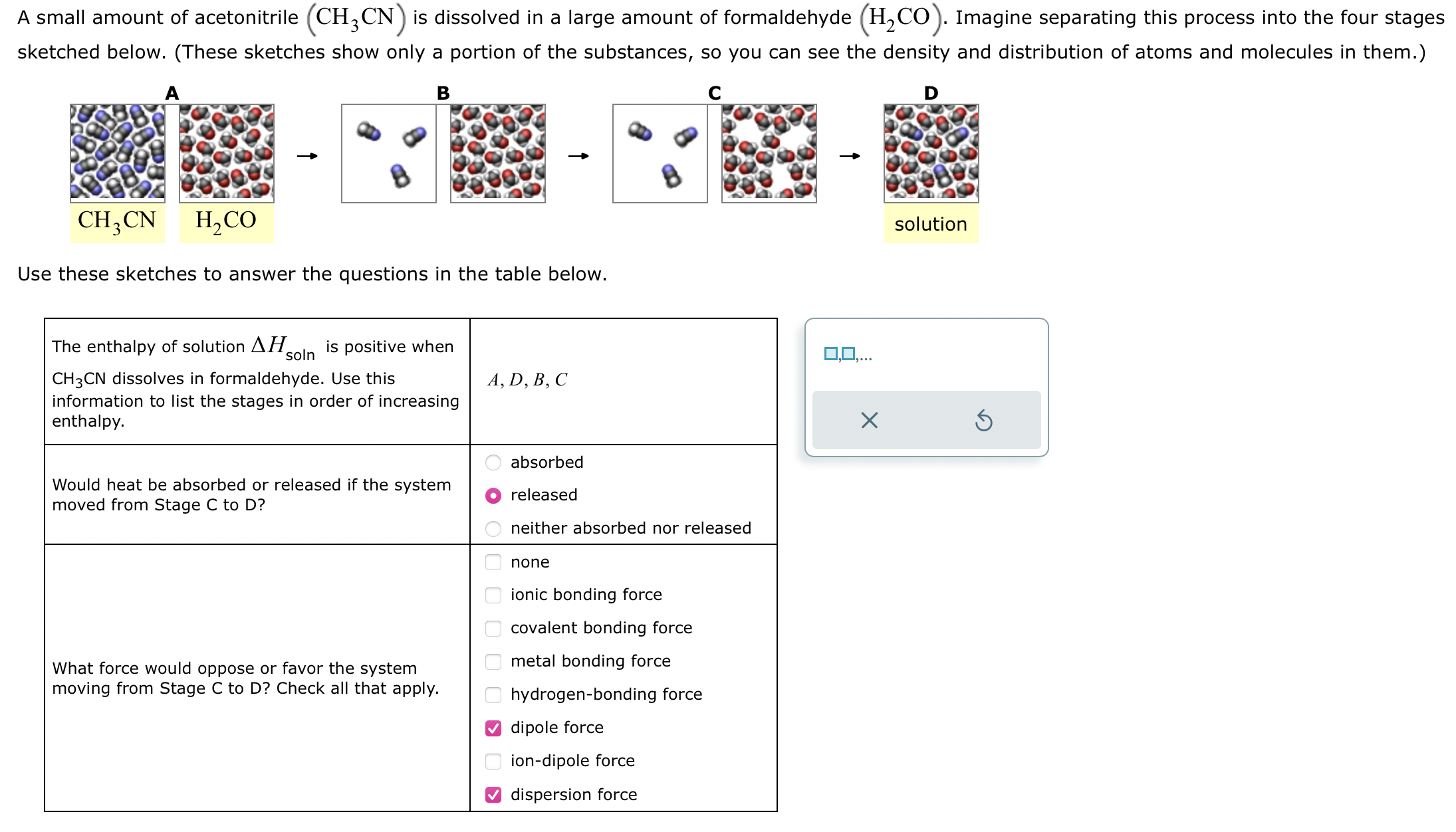
A small amount of acetonitrile CH 3 CN is dissolved in a large amount of formaldehyde H 2 CO . Imagine separating this process into
A small amount of acetonitrile CHCN is dissolved in a large amount of formaldehyde HCO Imagine separating this process into the four stages sketched below. These sketches show only a portion of the substances, so you can see the density and distribution of atoms and molecules in them.
A B C D
CHCN HCO solution
Use these sketches to answer the questions in the table below.
The enthalpy of solution Delta Hsoln is positive when CHCN dissolves in formaldehyde. Use this information to list the stages in order of increasing enthalpy.
Would heat be absorbed or released if the system moved from Stage C to D
absorbed
released
neither absorbed nor released
What force would oppose or favor the system moving from Stage C to D Check all that apply.
none
ionic bonding force
covalent bonding force
metal bonding force
hydrogenbonding force
dipole force
iondipole force
dispersion forceA small amount of acetonitrile is dissolved in a large amount of formaldehyde Imagine separating this process into the four stages
sketched below. These sketches show only a portion of the substances, so you can see the density and distribution of atoms and molecules in them.
Use these sketches to answer the questions in the table below.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


