Answered step by step
Verified Expert Solution
Question
1 Approved Answer
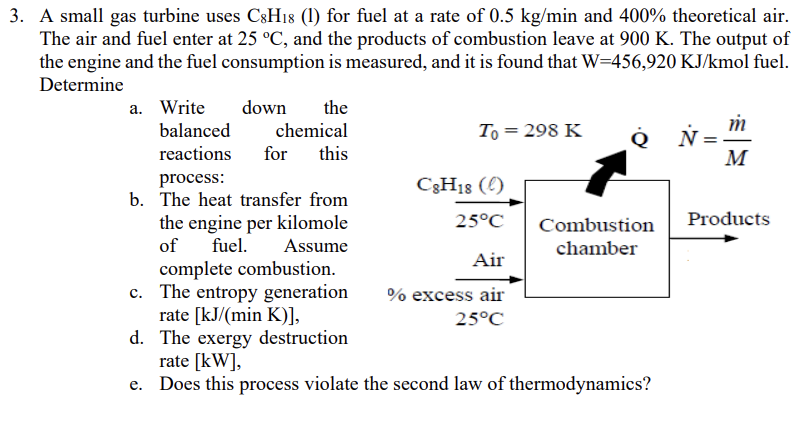
A small gas turbine uses C 8 H 1 8 ( l ) for fuel at a rate of 0 . 5 k g m
A small gas turbine uses l for fuel at a rate of and theoretical air.
The air and fuel enter at and the products of combustion leave at The output of
the engine and the fuel consumption is measured, and it is found that mol fuel.
Determine
a Write down the
balanced chemical
reactions for this
process:
b The heat transfer from
the engine per kilomole
of fuel. Assume
complete combustion.
c The entropy generation
rate
d The exergy destruction
rate
e Does this process violate the second law of thermodynamics?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


