Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A small - scale experiment requires the preparation Of a 4 0 mole % sulfuric acid solution from pure water and 8 0 mole %
A smallscale experiment requires the preparation Of a mole sulfuric acid solution from pure water and mole sulfuric acid. The mixture is to be prepared by metering one fluid at a constant molar rate into a tank containing the second fluid. Cooling is to be provided to maintain the bath at a constant temperature.
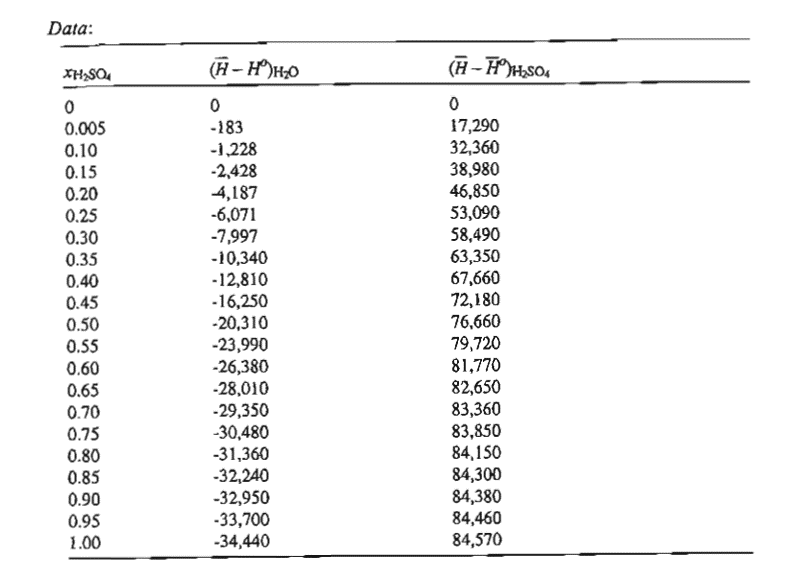
At the temperature of operation, K partial molar enthalpies are listed below Unternational Critical Tables Vol. McGrawHill, New York, p Ihe term HO HS refers to a state of infinite dilution ie where the mole fraction acid approaches zero BOH is the enthalpy of pure water at K x is mole fraction and enthalpies are expressed in Jmol
a Which fluid should be charged to the reactor first in order to minimize the rate of heat release?
b What is the peak differential heat load per mole of fluid added?
c What is the total heat of mixing per mole of final solution for both methods?
Parts ac for Case II Addintg mol acid to pure water
Parts ac with numerical answers for both cases.
Parts ac using Figure of Sandler.
Comment on the difference between the two casesData:Data:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


