Question
A Soft plastic bottle, filled with water of density 1 gm/cc, carries an inverted glass test-tube with some air (ideal gas) trapped as shown
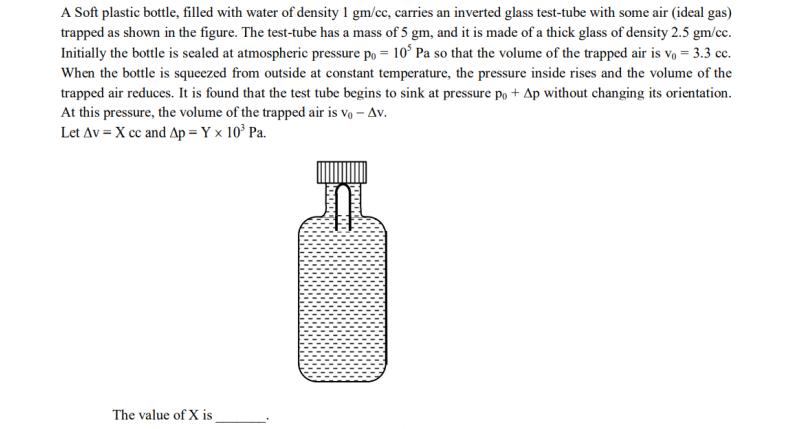
A Soft plastic bottle, filled with water of density 1 gm/cc, carries an inverted glass test-tube with some air (ideal gas) trapped as shown in the figure. The test-tube has a mass of 5 gm, and it is made of a thick glass of density 2.5 gm/cc. Initially the bottle is sealed at atmospheric pressure po = 105 Pa so that the volume of the trapped air is vo= 3.3 cc. When the bottle is squeezed from outside at constant temperature, the pressure inside rises and the volume of the trapped air reduces. It is found that the test tube begins to sink at pressure po + Ap without changing its orientation. At this pressure, the volume of the trapped air is Vo - Av. Let Av = X cc and Ap= Y x 10 Pa. The value of X is
Step by Step Solution
3.42 Rating (171 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Momentum Heat And Mass Transfer
Authors: James Welty, Gregory L. Rorrer, David G. Foster
6th Edition
1118947460, 978-1118947463
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



