Question: A solution containing a mixture of metal cations was treated with dilute HCI and no precipitate formed. Next, HS was bubbled through the acidic
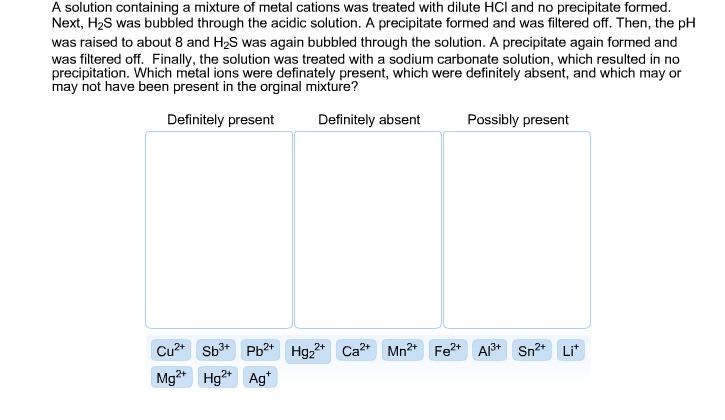
A solution containing a mixture of metal cations was treated with dilute HCI and no precipitate formed. Next, HS was bubbled through the acidic solution. A precipitate formed and was filtered off. Then, the pH was raised to about 8 and HS was again bubbled through the solution. A precipitate again formed and was filtered off. Finally, the solution was treated with a sodium carbonate solution, which resulted in no precipitation. Which metal ions were definately present, which were definitely absent, and which may or may not have been present in the orginal mixture? Definitely present Definitely absent Cu+ Sb+ Pb2+ Hg+ 2+ Mg2+ Hg2+ Ag* Possibly present Ca+ Mn+ Fe+ Al+ Sn+ Lit
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
The detailed answer for the above question is provided below DEFINITELY PRESENT None DEFINI... View full answer

Get step-by-step solutions from verified subject matter experts


