Question
A solution of carbon tetrachloride and carbon disulfide containing 50 wt% each is to be continuously fractionated at standard atmospheric pressure at the rate 4000
A solution of carbon tetrachloride and carbon disulfide containing 50 wt% each is to be continuously fractionated at standard atmospheric pressure at the rate 4000 kg/h. The distillate product is to contain 95wt % carbon disulfide, the residue 0.05%. The feed will be 30 mol % vaporized before it enters the tower. A total condenser will be used, and reflux will be returned at the bubble point. Equilibrium data, x, y = mole fraction CS2.
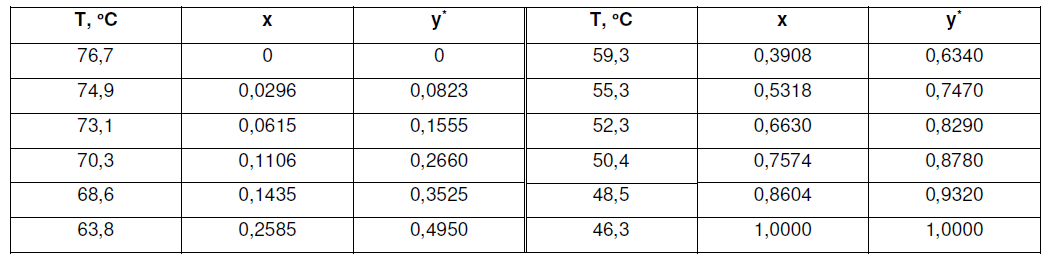
find:
a. Determine the product rates, kg/h.
b. Determine the minimum reflux ratio.
c. Calculate the minimum number of ideal plates.
d. Determine the number of theoretical trays required at a reflux ratio equal to twice the minimum and the position of the feed tray.
e. Using the temperature of the distillate as the base temperature, calculate the enthalpy of the feed, products and steam entering the products and steam entering the condenser. Calculate the heat loads of the condenser and reboiler.

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


