A solution of carbon tetrachloride and carbon disulfide containing 50 wt.% each is to be continuously fractionated at standard atmospheric pressure at the rate
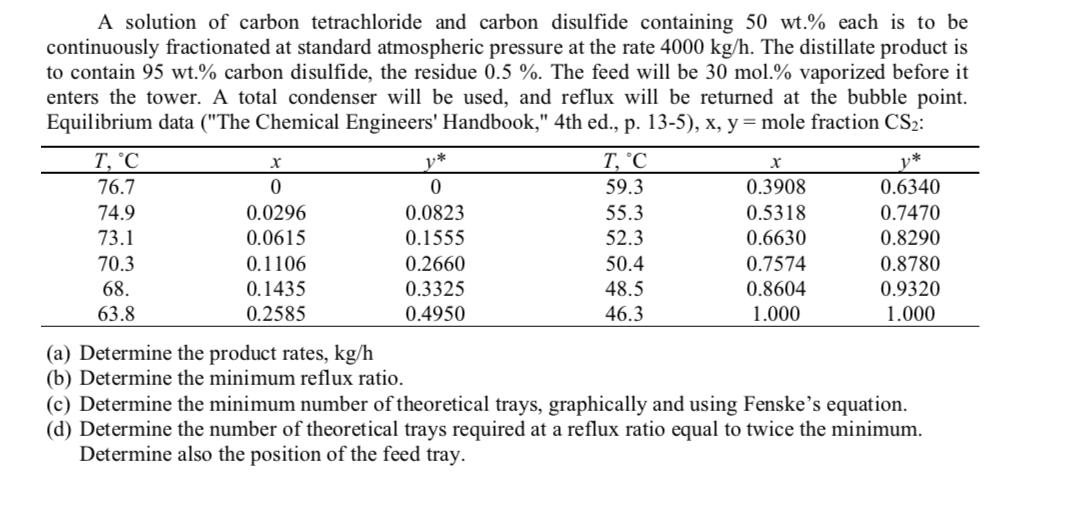
A solution of carbon tetrachloride and carbon disulfide containing 50 wt.% each is to be continuously fractionated at standard atmospheric pressure at the rate 4000 kg/h. The distillate product is to contain 95 wt.% carbon disulfide, the residue 0.5 %. The feed will be 30 mol.% vaporized before it enters the tower. A total condenser will be used, and reflux will be returned at the bubble point. Equilibrium data ("The Chemical Engineers' Handbook," 4th ed., p. 13-5), x, y = mole fraction CS2: T, 'C y* T, C v* 76.7 59.3 0.3908 0.6340 74.9 0.0296 0.0823 55.3 0.5318 0.7470 73.1 0.0615 0.1555 52.3 0.6630 0.8290 70.3 0.1106 0.2660 50.4 0.7574 0.8780 68. 0.1435 0.3325 48.5 0.8604 0.9320 63.8 0.2585 0.4950 46.3 1.000 1.000 (a) Determine the product rates, kg/h (b) Determine the minimum reflux ratio. (c) Determine the minimum number of theoretical trays, graphically and using Fenske's equation. (d) Determine the number of theoretical trays required at a reflux ratio equal to twice the minimum. Determine also the position of the feed tray.
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Important note To apply McCabeThiel...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


