Answered step by step
Verified Expert Solution
Question
1 Approved Answer
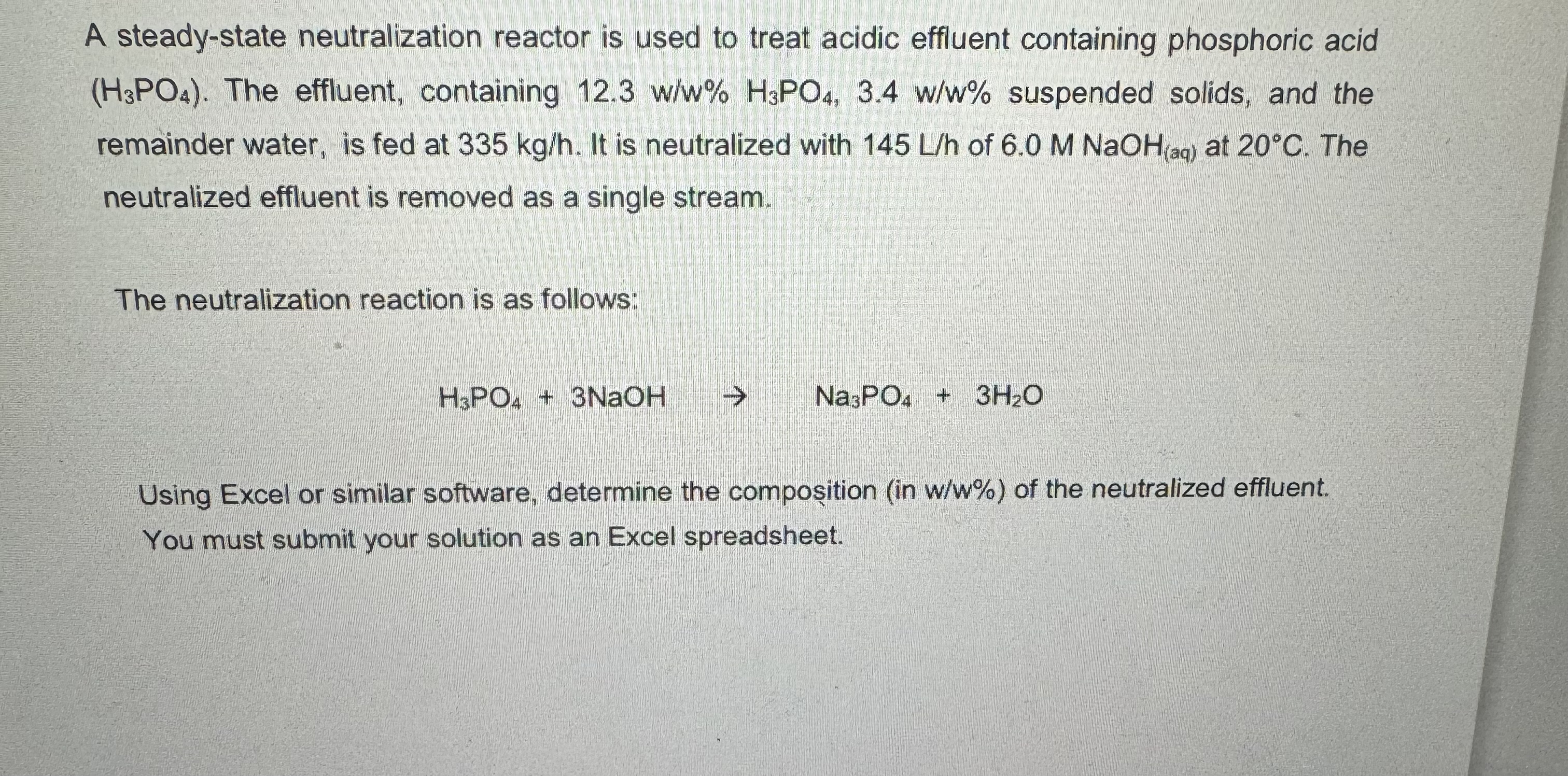
A steady - state neutralization reactor is used to treat acidic effluent containing phosphoric acid ( H 3 P O 4 ) . The effluent,
A steadystate neutralization reactor is used to treat acidic effluent containing phosphoric acid The effluent, containing suspended solids, and the remainder water, is fed at It is neutralized with of at The neutralized effluent is removed as a single stream.
The neutralization reaction is as follows:
NaOH
Using Excel or similar software, determine the composition in ww of the neutralized effluent. You must submit your solution as an Excel spreadsheet.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


