Question
A stoichiometric mixture of ethane and air reacts at 15C. Using the following values for Cp and values given in Table 2.5, determine the
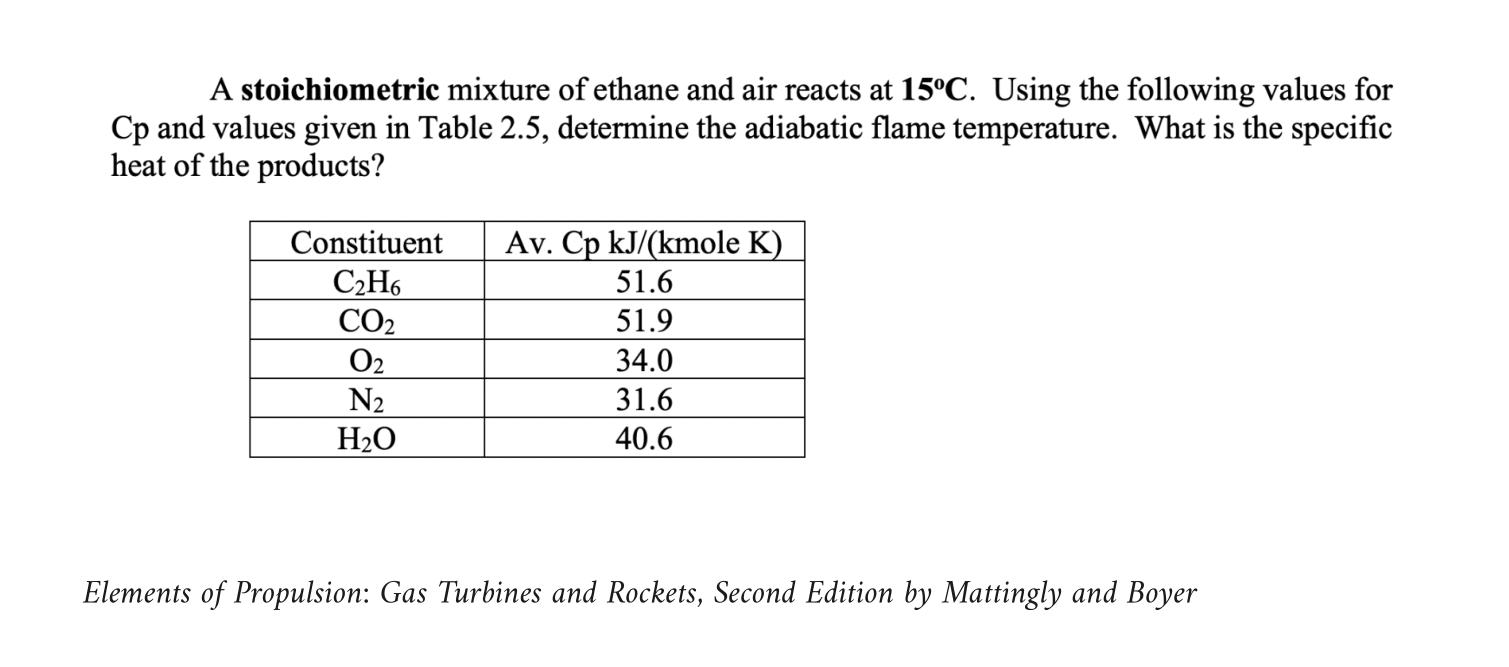
A stoichiometric mixture of ethane and air reacts at 15C. Using the following values for Cp and values given in Table 2.5, determine the adiabatic flame temperature. What is the specific heat of the products? Constituent CH6 CO 0 N HO Av. Cp kJ/(kmole K) 51.6 51.9 34.0 31.6 40.6 Elements of Propulsion: Gas Turbines and Rockets, Second Edition by Mattingly and Boyer
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
To determine the adiabatic flame temperature and the specific heat of the products we need to use the given values for Cp and perform some calculations First lets calculate the heat of reaction for th...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
4th edition
978-1118431221, 9781119192138, 1118431227, 1119192137, 978-1119498759
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



