Answered step by step
Verified Expert Solution
Question
1 Approved Answer
. A stream consisting of a mixture of N2 and water vapor, at 98.0 C and 5200.0 mm Hg, enters a compressor. This inlet stream
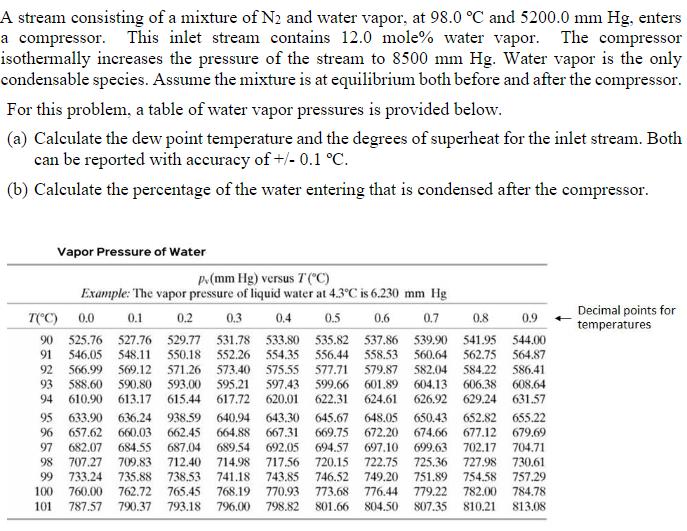 .
.
A stream consisting of a mixture of N2 and water vapor, at 98.0 C and 5200.0 mm Hg, enters a compressor. This inlet stream contains 12.0 mole% water vapor. The compressor isothermally increases the pressure of the stream to 8500 mm Hg. Water vapor is the only condensable species. Assume the mixture is at equilibrium both before and after the compressor. For this problem, a table of water vapor pressures is provided below. (a) Calculate the dew point temperature and the degrees of superheat for the inlet stream. Both can be reported with accuracy of +/- 0.1 C. (b) Calculate the percentage of the water entering that is condensed after the compressor. Vapor Pressure of Water p.(mm Hg) versus T("C) Example: The vapor pressure of liquid water at 4.3C is 6.230 mm Hg 0.1 0.3 0.9 Decimal points for temperatures T(*C) 0.0 0.2 0.4 0.5 0.6 0.7 0.8 90 525.76 527.76 529.77 531.78 548,11 533.80 535.82 537.86 539.90 541.95 544.00 91 546.05 550.18 552.26 554.35 556.44 558.53 560.64 562.75 564.87 575.55 597.43 617.72 620.01 582.04 604.13 626.92 629.24 631.57 584.22 586.41 606.38 608.64 92 566.99 569.12 571.26 573.40 577.71 579.87 93 588.60 94 610,90 593.00 615.44 595.21 599.66 622.31 601.89 624,61 590.80 613.17 938.59 662.45 640.94 643.30 667.31 648.05 650.43 674.66 697.10 699.63 725.36 751.89 779.22 652.82 655.22 95 657.62 633.90 636.24 645.67 669.75 96 660.03 664.88 672.20 677.12 679.69 692.05 717.56 702.17 727.98 730.61 754.58 757.29 97 682.07 684.55 687.04 689.54 694.57 704.71 707.27 709,83 735.88 762.72 712.40 714,98 741.18 768,19 98 720,15 722.75 99 733.24 738.53 743.85 746.52 749.20 765.45 770,93 798.82 100 760.00 773.68 776.44 782.00 784.78 101 787.57 790.37 793.18 796.00 801.66 804.50 807.35 810.21 813.08
Step by Step Solution
★★★★★
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Degree of superheeat is the extra temperature in addition to saturation temperature Part 1 At inlet ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


