Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A student performed an experiment to calculate the density of 10% aqueous solution of sodium chloride, NaCl and collects the following data. Complete the data/result
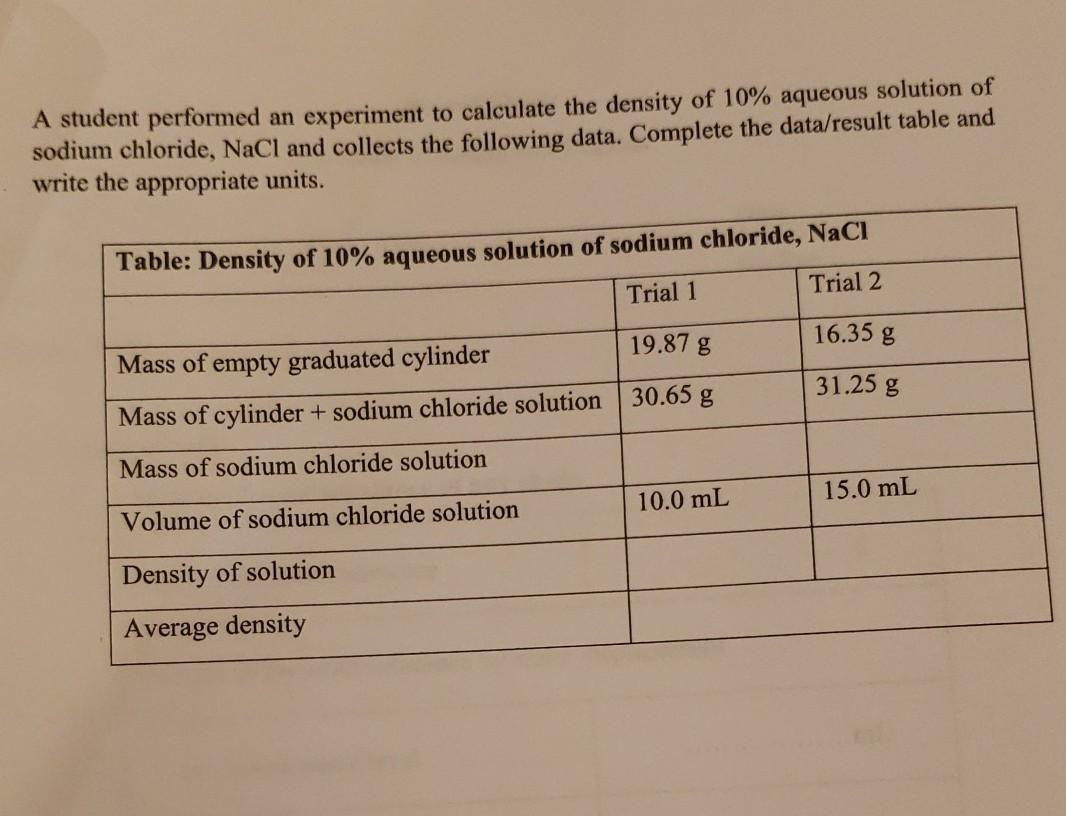
A student performed an experiment to calculate the density of 10% aqueous solution of sodium chloride, NaCl and collects the following data. Complete the data/result table and write the appropriate units. Table: Density of 10% aqueous solution of sodium chloride, NaCI Trial 1 Trial 2 19.87 g 16.35 g Mass of empty graduated cylinder Mass of cylinder + sodium chloride solution 30.65 g 31.25 g Mass of sodium chloride solution 10.0 mL 15.0 mL Volume of sodium chloride solution Density of solution Average density
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


