A student performed three CHCl2 layer titrations in Part 2 using a standard sodium thiosulfate solution with a concentration of 0.01153 M. They reported
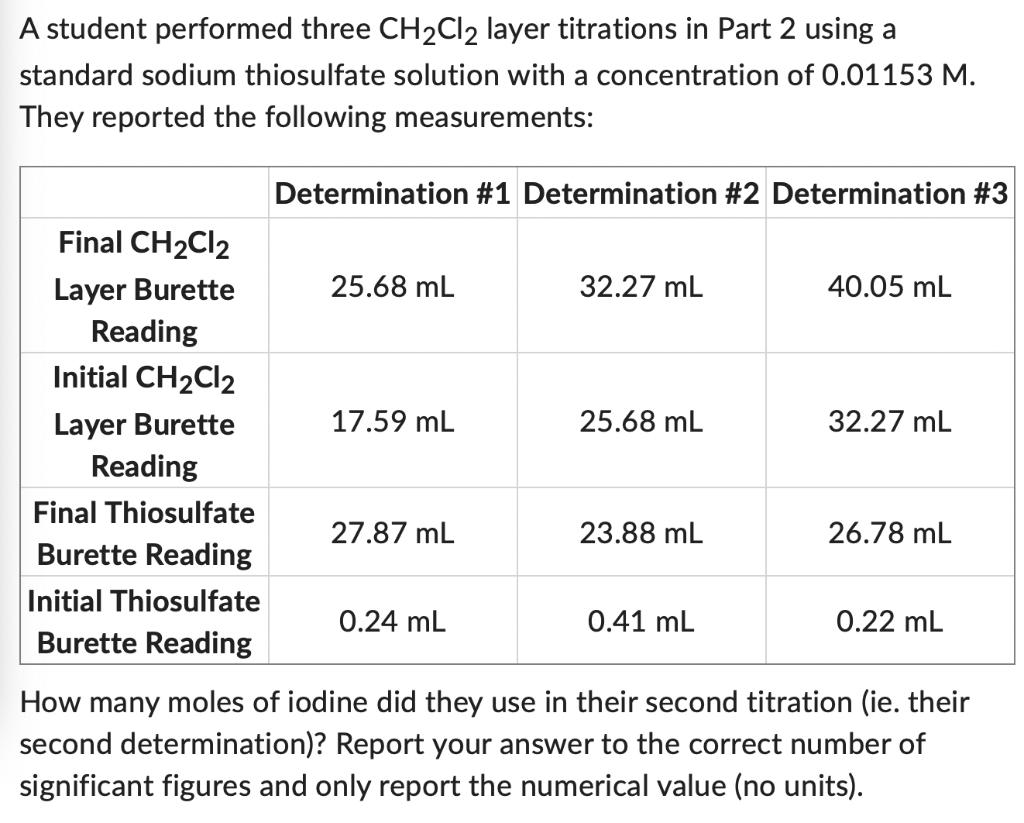
A student performed three CHCl2 layer titrations in Part 2 using a standard sodium thiosulfate solution with a concentration of 0.01153 M. They reported the following measurements: Final CHCl2 Layer Burette Reading Initial CHCl2 Layer Burette Reading Final Thiosulfate Burette Reading Initial Thiosulfate Burette Reading Determination #1 Determination #2 Determination #3 25.68 mL 17.59 mL 27.87 mL 0.24 mL 32.27 mL 25.68 mL 23.88 mL 0.41 mL 40.05 mL 32.27 mL 26.78 mL 0.22 mL How many moles of iodine did they use in their second titration (ie. their second determination)? Report your answer to the correct number of significant figures and only report the numerical value (no units).
Step by Step Solution
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Step 11 The number of moles of iodine used in the second titration can be calculated using the formu...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


