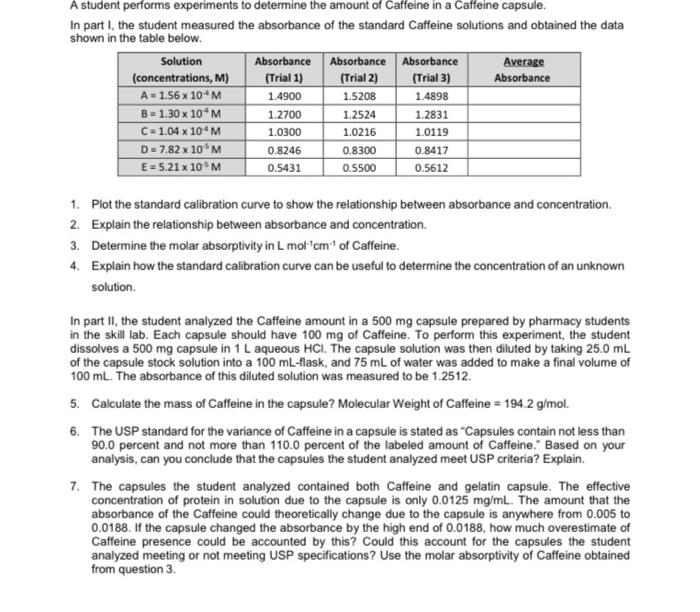
A student performs experiments to determine the amount of Caffeine in a Caffeine capsule. In part I, the student measured the absorbance of the standard Caffeine solutions and obtained the data shown in the table below. Solution Absorbance Absorbance Absorbance Average (concentrations, M) (Trial 1) (Trial 2) (Trial 3) Absorbance A = 1.56 x 10 M 1.4900 1.5208 1.4898 B = 1.30 x 10 M 1.2700 1.2524 1.2831 C = 1.04 x 10^M 1.0300 1.0216 1.0119 D = 7.82 x 10 M 0.8246 0.8300 0.8417 E = 5.21 x 10 M 0.5431 0.5500 0.5612 1. Plot the standard calibration curve to show the relationship between absorbance and concentration. 2. Explain the relationship between absorbance and concentration. 3. Determine the molar absorptivity in L mol'cm' of Caffeine. 4. Explain how the standard calibration curve can be useful to determine the concentration of an unknown solution In part II, the student analyzed the Caffeine amount in a 500 mg capsule prepared by pharmacy students in the skill lab. Each capsule should have 100 mg of caffeine. To perform this experiment, the student dissolves a 500 mg capsule in 1 L aqueous HCI. The capsule solution was then diluted by taking 25.0 mL of the capsule stock solution into a 100 mL-flask, and 75 mL of water was added to make a final volume of 100 ml. The absorbance of this diluted solution was measured to be 1.2512. 5. Calculate the mass of Caffeine in the capsule? Molecular weight of Caffeine = 194.2 g/mol. 6. The USP standard for the variance of Caffeine in a capsule is stated as "Capsules contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of Caffeine. Based on your analysis, can you conclude that the capsules the student analyzed meet USP criteria? Explain. 7. The capsules the student analyzed contained both Caffeine and gelatin capsule. The effective concentration of protein in solution due to the capsule is only 0.0125 mg/ml. The amount that the absorbance of the Caffeine could theoretically change due to the capsule is anywhere from 0.005 to 0.0188. If the capsule changed the absorbance by the high end of 0.0188, how much overestimate of Caffeine presence could be accounted by this? Could this account for the capsules the student analyzed meeting or not meeting USP specifications? Use the molar absorptivity of Caffeine obtained from question 3