Question: A student was given an unknown metal. The student determined that the mass of the metal was 30.2 g. The student placed the metal in
A student was given an unknown metal. The student determined that the mass of the metal was 30.2 g. The student placed the metal in a graduated cylinder filled with 20.0 mL of water. The metal increased the volume of water to 22.9 mL. Calculate the density of the metal and determine the identity of the metal using the table below. 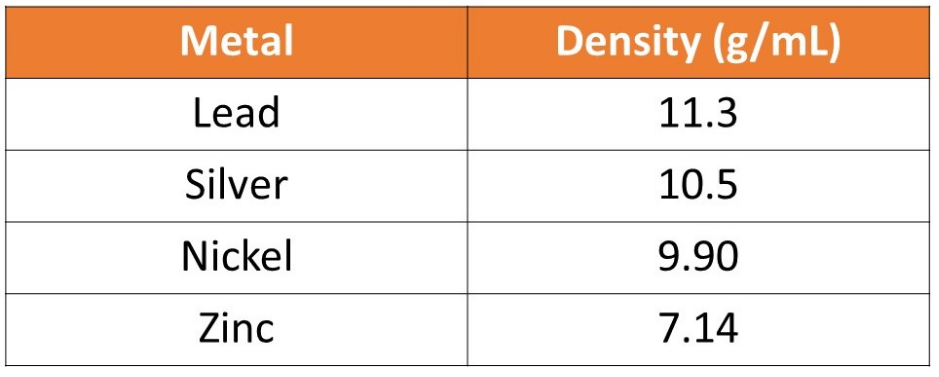
Metal Lead Silver Nickel Zinc Density (g/mL) 11.3 10.5 9.90 7.14
Step by Step Solution
3.39 Rating (146 Votes )
There are 3 Steps involved in it
To find the density of the unknown metal we can use the formula for ... View full answer

Get step-by-step solutions from verified subject matter experts


