Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) Suppose the student wanted to determine the order of the reaction. Describe what the student should do to analyze this data. b) Qualitatively, how
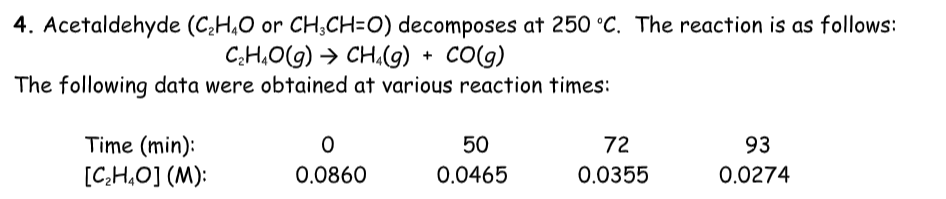
a) Suppose the student wanted to determine the order of the reaction. Describe what the student should do to analyze this data.
b) Qualitatively, how would the student know if the reaction was consistent with first-order or second-order kinetics?
c) The student used the data as suggested in parts a and b and determined that the reaction was first order in C 2 H 4 O. What is the rate constant (with units) for the reaction?
d) How long must we wait until 90 % of the starting material has disappeared to form products?
4. Acetaldehyde (C2H4O or CH3CH=O) decomposes at 250C. The reaction is as follows: C2H4O(g)CH4(g)+CO(g) The following data were obtained at various reaction timesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


