Answered step by step
Verified Expert Solution
Question
1 Approved Answer
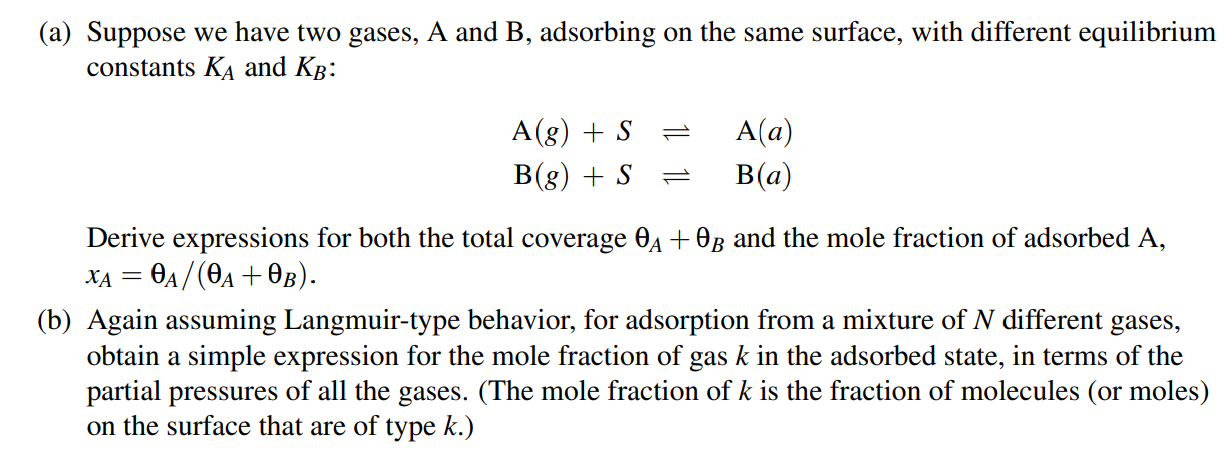
( a ) Suppose we have two gases, A and B , adsorbing on the same surface, with different equilibrium constants K A and K
a Suppose we have two gases, A and B adsorbing on the same surface, with different equilibrium
constants and :
Derive expressions for both the total coverage and the mole fraction of adsorbed A
b Again assuming Langmuirtype behavior, for adsorption from a mixture of different gases,
obtain a simple expression for the mole fraction of gas in the adsorbed state, in terms of the
partial pressures of all the gases. The mole fraction of is the fraction of molecules or moles
on the surface that are of type
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


