Question
A gas mixture at 500C with 6.4% CO2, 0.2% O2, 40.0% CO, 50.8% H2 (% mol), (the remainder is N2) is burned with 40%
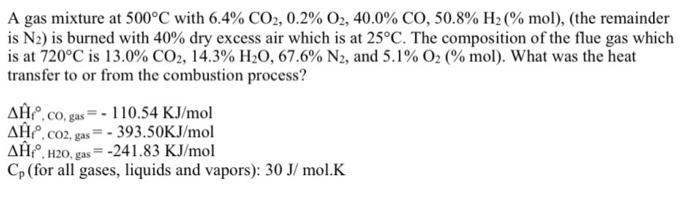
A gas mixture at 500C with 6.4% CO2, 0.2% O2, 40.0% CO, 50.8% H2 (% mol), (the remainder is N2) is burned with 40% dry excess air which is at 25C. The composition of the flue gas which is at 720C is 13.0% CO2, 14.3% H20, 67.6% N2, and 5.1% 02 (% mol). What was the heat transfer to or from the combustion process? AP, co, gas= - 110.54 KJ/mol AE. co2, gas= - 393.50KJ/mol A, H20, gas=-241.83 KJ/mol Cp (for all gases, liquids and vapors): 30 J/ mol.K
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
The heat of a reaction can be defined as the enthalpy change of the system which is the sum of the s...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Vector Mechanics for Engineers Statics and Dynamics
Authors: Ferdinand Beer, E. Russell Johnston, Jr., Elliot Eisenberg, William Clausen, David Mazurek, Phillip Cornwell
8th Edition
73212229, 978-0073212227
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



