Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A system that is not at equilibrium is found to contain 0.100M of (aq),0.100M of B(aq), and 0.140M of C(aq). Note that each of these
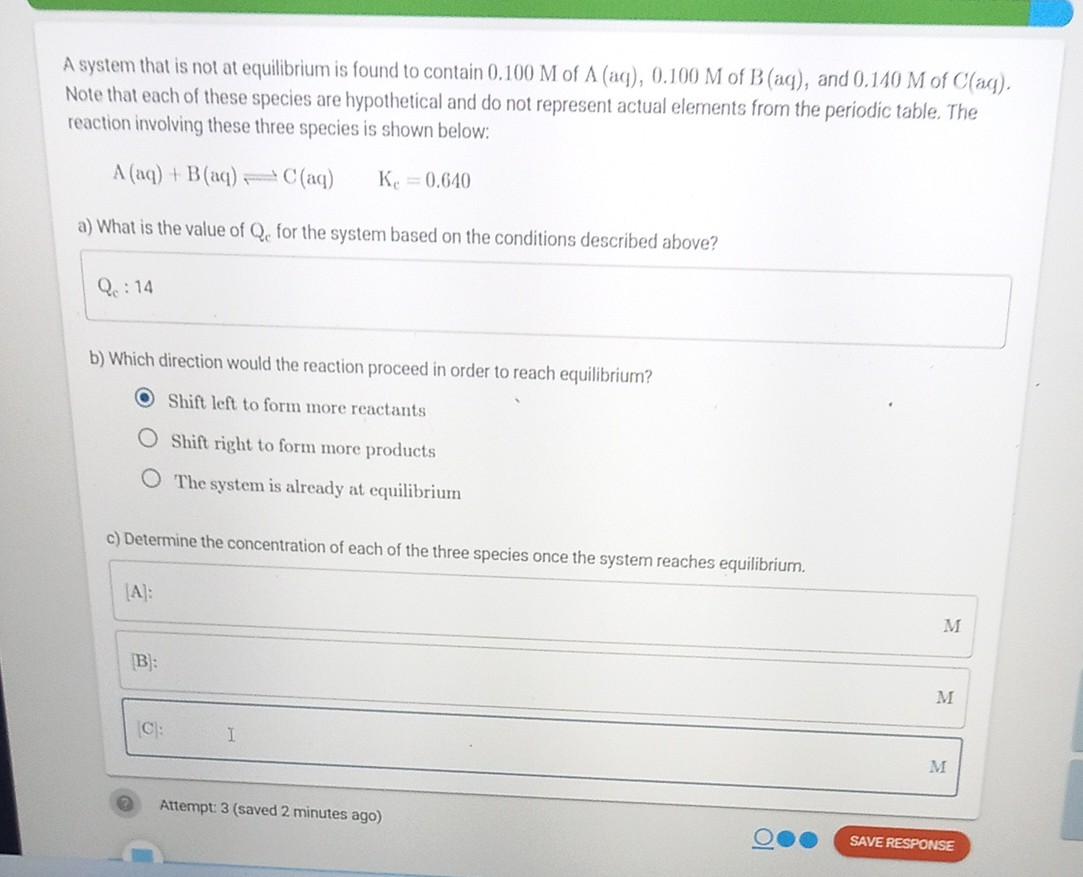
A system that is not at equilibrium is found to contain 0.100M of (aq),0.100M of B(aq), and 0.140M of C(aq). Note that each of these species are hypothetical and do not represent actual elements from the periodic table. The reaction involving these three species is shown below: A(aq)+B(aq)C(aq)Kc=0.640 a) What is the value of Qc for the system based on the conditions described above? b) Which direction would the reaction proceed in order to reach equilibrium? Shift left to form more reactants Shift right to form more products The system is already at equilibrium c) Determine the concentration of each of the three species once the system reaches equilibrium. [A]: (B): M [C] : Attempt: 3 (saved 2 minutes ago)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


