Question
A tank contains 1800 kg of 12% saline solution of ammonium nitrate (NH4NO3). The tank receives two continuous streams of solution of the same salt
A tank contains 1800 kg of 12% saline solution of ammonium nitrate (NH4NO3).
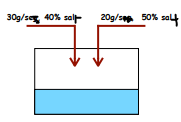
The tank receives two continuous streams of solution of the same salt at different concentrations, as shown in the diagram.
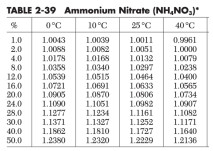
After a considerable time, both streams were closed and the density of the resulting solution was 1.1161g/cm3. If the entire process was carried out at 77 F, calculate,
a) The time (hours) that elapsed, from the time there were 1800 kg of solution, until the taps of the streams were closed. the taps of the streams were closed.
b) The amount (kg) of total salt dissolved in the final solution.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


