Answered step by step
Verified Expert Solution
Question
1 Approved Answer
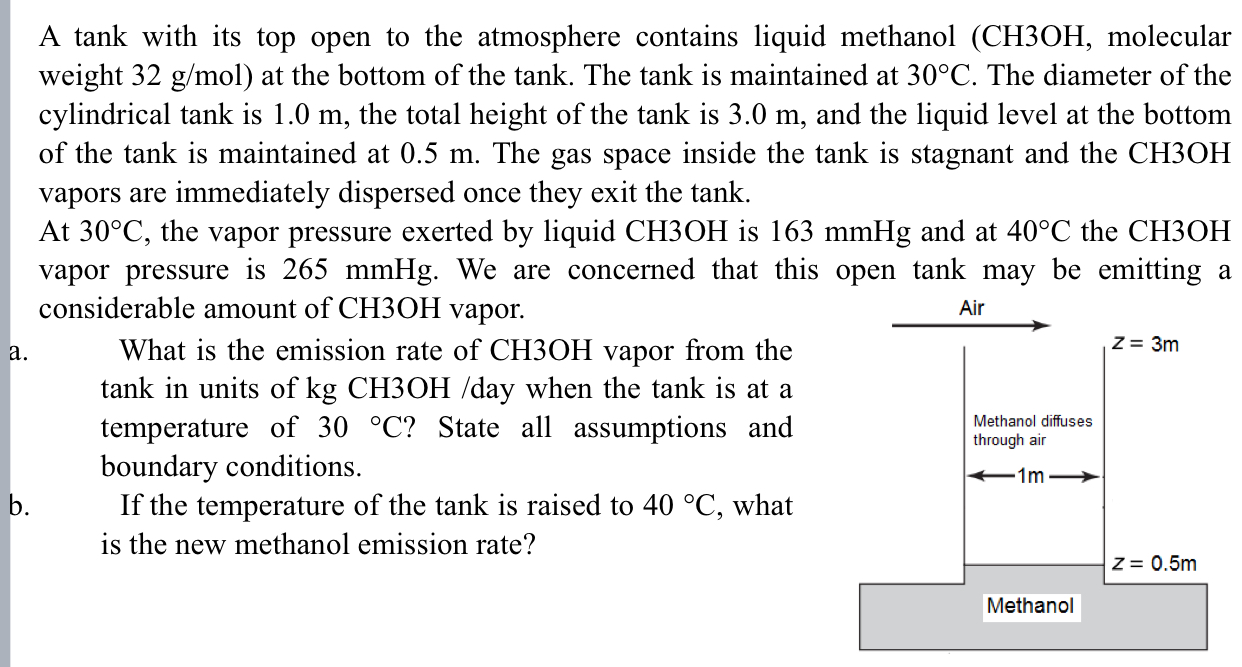
A tank with its top open to the atmosphere contains liquid methanol , molecular weight 3 2 g m o l ) at the bottom
A tank with its top open to the atmosphere contains liquid methanol molecular weight at the bottom of the tank. The tank is maintained at The diameter of the cylindrical tank is the total height of the tank is and the liquid level at the bottom of the tank is maintained at The gas space inside the tank is stagnant and the vapors are immediately dispersed once they exit the tank.
At the vapor pressure exerted by liquid is and at the vapor pressure is We are concerned that this open tank may be emitting a considerable amount of vapor.
a What is the emission rate of vapor from the tank in units of day when the tank is at a temperature of State all assumptions and boundary conditions.
b If the temperature of the tank is raised to what is the new methanol emission rate?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


