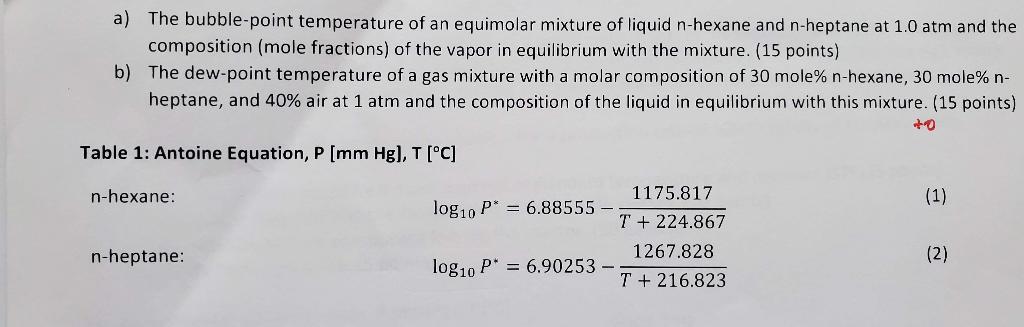
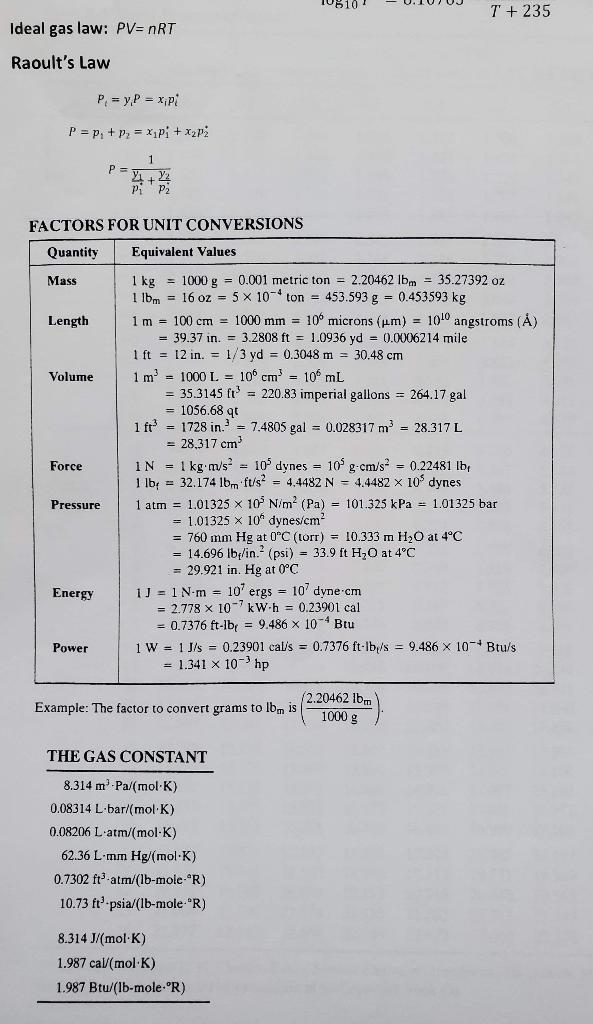
a) The bubble-point temperature of an equimolar mixture of liquid n-hexane and n-heptane at 1.0 atm and the composition (mole fractions) of the vapor in equilibrium with the mixture. (15 points) b) The dew-point temperature of a gas mixture with a molar composition of 30 mole% n-hexane, 30 mole% n- heptane, and 40% air at 1 atm and the composition of the liquid in equilibrium with this mixture. (15 points) Table 1: Antoine Equation, P [mm Hg), T [C] n-hexane: (1) 1175.817 log10 P* = 6.88555 - T + 224.867 1267.828 log10 P* = 6.90253 - T + 216.823 n-heptane: (2) T + 235 Ideal gas law: PV=nRT Raoult's Law P = y.P = x.pl P = P + P = x + x 1 1 P= + +2 P2 FACTORS FOR UNIT CONVERSIONS Quantity Equivalent Values Mass 1 kg = 1000 g = 0.001 metric ton = 2.20462 Ibm = 35.27392 oz 1 lbm = 16 oz = 5 x 10-4 ton = 453.593 g = 0.453593 kg Length 1m = 100 cm = 1000 mm = 10 microns (um) = 100 angstroms (8) = 39.37 in. = 3.2808 ft = 1.0936 yd = 0.0006214 mile 1 ft = 12 in. = 1/3 yd = 0.3048 m = 30.48 cm Volume 1 m = 1000 L = 10 cm - 10 mL = 35.3145 l = 220.83 imperial gallons = 264.17 gal = 1056.68 qt ift = 1728 in. = 7.4805 gal = 0.028317 m = 28.317 L = 28.317 cm Force IN = 1 kgm/s = 10% dynes = 109g.cm/s2 = 0.22481 lb 1 lb = 32.174 lbm ft/s = 4.4482 N = 4.4482 x 10% dynes Pressure 1 atm = 1.01325 x 10 N/m (Pa) = 101.325 kPa = 1.01325 bar = 1.01325 X 10" dynes/cm? = 760 mm Hg at 0C (torr) = 10.333 m H2O at 4C = 14.696 lb/in. (psi) = 33.9 ft H20 at 4C = 29.921 in. Hg at 0C Energy 1 = 1 Nm = 10' ergs = 107 dyne cm = 2.778 x 10-7 kW.h = 0.23901 cal = 0.7376 ft-lby = 9.486 x 10 Btu Power 1 W = 1J/s = 0.23901 calls = 0.7376 ft-lb/s = 9.486 X 10- Btu/s = 1.341 x 10-3 hp (2.20462 lbm Example: The factor to convert grams to lbn is 1000 g THE GAS CONSTANT 8.314 m.Pa/(mol-K) 0.08314 Lbar/mol.K) 0.08206 L'atm/(mol-K) 62.36 L mm Hg/(mol.K) 0.7302 ft atm/(Ib-mole-"R) 10.73 ft.psia/(Ib-mole-R) 8.314 J/mol K) 1.987 cal/(mol.K) 1.987 Btu/(Ib-mole-'R) Table B.3 Vapor Pressure of Water pu(mm Hg) versus T (C) Example: The vapor pressure of liquid water at 4.3C is 6.230 mm Hg T(C) 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 -14 -13 -12 Ice -11 1.361 1.490 1.632 1.785 1.950 2.131 2.326 -10 1.348 1.477 1.617 1.769 1.934 2.122 2.306 2.515 2.742 2.987 -9 -8 -7 -6 2.537 1.336 1.464 1.602 1.753 1.916 2.093 2.285 2.493 2.718 2.962 3.225 3.509 3.816 4.147 4.504 1.324 1.450 1.588 1.737 1.899 2.075 2.266 2.472 2.695 2.937 3.198 3.480 3.785 4.113 4.467 1.312 1.437 1.574 1.722 1.883 2.057 2.246 2.450 2.672 2.912 3.171 3.451 3.753 4.079 4.431 1.300 1.424 1.559 1.707 1.866 2.039 2.226 2.429 2.649 2.887 3.144 3.422 3.722 4.045 4.395 1.288 1.411 1.546 1.691 1.849 2.021 2.207 2.408 2.626 2.862 3.117 3.393 3.691 4.012 4.359 1.276 1.399 1.532 1.676 1.833 2.003 2.187 2.387 2.603 2.838 3.091 3.364 3.660 3.979 4.323 1.264 1.386 1.518 1.661 1.817 1.985 2.168 2.367 2.581 2.813 3.065 3.336 3.630 3.946 4.287 1.253 1.373 1.504 1.646 1.800 1.968 2.149 2.346 2.559 2.790 3.039 3.308 3.599 3.913 4.252 2.765 3.013 3.280 3.568 3.880 4.217 4.579 -2 - 1 -0 3.252 3.539 3.848 4.182 4.542 Liquid water 0 1 2 3 4 4.647 4.998 5.370 5.766 6.187 4.820 5.181 5.565 5.973 6.408 4.750 5.107 5.486 5.889 6.318 6.775 7.259 7.775 8.323 8.905 5 6 7 8 9 4.613 4.962 5.332 5.725 6.144 6.589 7.062 7.565 8.100 8.668 9.271 9.910 10.588 11.305 12.065 4.579 4.926 5.294 5.685 6.101 6.543 7.013 7.513 8.045 8.609 9.209 9.844 10.518 11.231 11.987 12.788 13.634 14.530 15.477 16.477 17.535 18.650 19.827 21.068 22.377 10 11 12 13 14 4.681 5.034 5.408 5.807 6.230 6.681 7.160 7.669 8.211 8.786 9.395 10.042 10.728 11.453 12.223 13.037 13.898 14.809 15.772 16.789 6.635 7.111 7.617 8.155 8.727 9.333 9.976 10.658 11.379 12.144 12.953 13.809 14.715 15.673 16.685 17.753 18.880 20.070 21.324 22.648 4.715 5.070 5.447 5.848 6.274 6.728 7.209 7.722 8.267 8.845 9.458 10.109 10.799 11.528 12.302 13.121 13.987 14.903 15.871 16.894 17.974 19.113 20.316 21.583 22.922 9.521 10.176 10.870 11.604 12.382 13.205 14.076 14.997 15.971 16.999 4.785 5.144 5.525 5.931 6.363 6.822 7.309 7.828 8.380 8.965 9.585 10.244 10.941 11.680 12.462 13.290 14.166 15.092 16.771 17.105 18.197 19.349 20.565 21.845 23.198 4.855 4.890 5.219 5.256 5.605 5.645 6.015 6.058 6.453 6.498 6.917 6.965 7.411 7.462 7.936 7.990 8.494 8.551 9.086 9.147 9.714 9.779 10.380 10.449 11.085 11.158 11.833 11.910 12.624 12.706 13.461 13.547 14.347 14.438 15.284 15.380 16.272 16.374 17.319 17.427 18.422 18.536 19.587 19.707 20.815 20.941 22.110 22.243 23.476 23.616 15 16 17 18 19 6.869 7.360 7.882 8.437 9.025 9.649 10.312 11.013 11.756 12.543 13.375 14.256 15.188 16.171 17.212 18.309 19.468 20.690 21.977 23.337 12.870 13.721 14.622 15.575 16.581 17.644 18.765 19.948 21.196 22.512 20 21 22 23 24 17.863 18.996 20.193 21.453 22.785 18.085 19.231 20.440 21.714 23.060 Table B.3 (Continued) T(C) 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 25 23.756 23.897 24.039 26 25.209 25.359 25.509 27 26.739 26.897 27.055 28 28.349 28.514 28.680 29 30.043 30.217 30.392 30 31.824 32.007 32.191 31 33.695 33.888 34.082 32 35.663 35.865 36.068 33 37.729 37.942 38.155 34 39.898 40.121 40.344 35 42.175 42.409 42.644 36 44.563 44.808 45.054 37 47.067 47.324 47.582 38 49.692 49.961 50.231 39 52.442 52.725 53.009 40 55.324 55.61 55.91 41 58.34 58.65 58.96 42 61.50 61.82 62.14 43 64.80 65.14 65.48 44 68.26 68.61 68.97 45 71.88 72.25 72.62 46 75.65 76.04 76.43 47 79.60 80.00 80.41 48 83.71 84.13 84.56 49 88.02 88.46 88.90 24.182 25.660 27.214 28.847 30.568 32.376 34.276 36.272 38.369 40.569 42.880 45.301 47.841 50.502 53.294 56.21 24.326 25.812 27.374 29.015 30.745 32.561 34.471 36.477 33.584 40.796 43.117 45.549 48.102 50.774 $3.580 56.51 59.58 62.80 66.16 69.69 73.36 77.21 81.23 85.42 89.79 24.471 24.617 25.964 26.117 27.535 27.696 29.184 29.354 30.923 31.102 32.747 32.934 34.667 34.864 36.683 36.891 38.801 38.018 41.023 41.251 43.355 43.595 45.799 46.050 48.364 48.627 51.048 51.323 53.867 54.156 56.81 57.11 59.90 60.22 63.13 63.46 66.51 66.86 70.05 70.41 73.74 74.12 77.60 78.00 81.64 82.05 85.85 86.28 90.24 90.69 24.764 24.912 25.060 26.271 26.426 26.582 27.858 28.021 28.185 29.525 29.697 29.870 31.281 31.461 31.642 33.122 33.312 33.503 35.062 35.261 35.462 37.099 37.308 37.518 39.237 39.457 39.677 41.480 41.710 41.942 43.836 44.078 44.320 46,302 46.556 46.811 48.891 49.157 49.424 51.600 51.879 52.160 54.446 54.737 55.030 57.41 57.72 58.03 60.54 60.86 61.18 63.79 64.12 64.46 67.21 67.56 67.91 70.77 71.14 71.51 74.50 74.88 75.26 78.40 78.80 79.20 82.46 82.87 83.29 86.71 87.14 87.58 91.14 91.59 92.05 59.27 62.47 65.82 69.33 72.99 76.82 80.82 84.99 89 34 T(C) 0 1 2 3 4 5 6 7 8 9 50 60 70 80 92.51 149.38 233.7 355.1 97.20 156.43 243.9 369.7 102.09 163.77 254.6 384.9 107.20 171.38 265.7 400.6 112.51 179.31 277.2 416.8 118.04 187.54 289.1 433.6 123.80 196.09 301.4 450.9 129.82 204.96 314.1 468.7 136.08 214.17 327.3 487.1 142.60 223.73 341.0 506.1 T(C) 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 531.78 90 91 92 93 94 95 96 97 98 99 100 101 525.76 546.05 566.99 588.60 610.90 633.90 657.62 682.07 707.27 733.24 760.00 787.57 527.76 529.77 548.11 350.18 569.12 571.26 590.80 593.00 613.17 615.44 636.24 938.59 660.03 662.45 684.55 687.04 709.83 712.40 735.88 738.53 762.72 765.45 790.37 793.18 533.80 552.26 554.35 573.40 575.55 595.21 597.43 617.72 620.01 640.94 643.30 664.88 667.31 689.54 692.05 714.98 717.56 741.18 743.85 768.19 770.93 796.00 798.82 535.82 556.44 577.71 599.66 622.31 645.67 669.75 694.57 720.15 746.52 773.68 801.66 537.86 $39.90 541.95 544.00 558.53 $60.64 562.75 564.87 $79.87 582.04 584.22 586.41 601.89 604.13 606.38 608.64 624.61 626.92 629.24 631.57 648.05 650.43 652.82 655.22 672.20 674.66 677.12 679.69 697.10 699.63 702.17 704.71 722.75 725.36 727.98 730.61 749.20 751.89 754.58 757.29 776.44 779.22 782.00 784.78 804.50 807.35 810.21 813.08










