Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) The dimerisation of 1,3-butadiene, C4H6 in the gaseous phase is a second order reaction. 2C4H6(g)C8H12(g)H=150kJmol1 [A]6M An experiment was carried out at 300C to
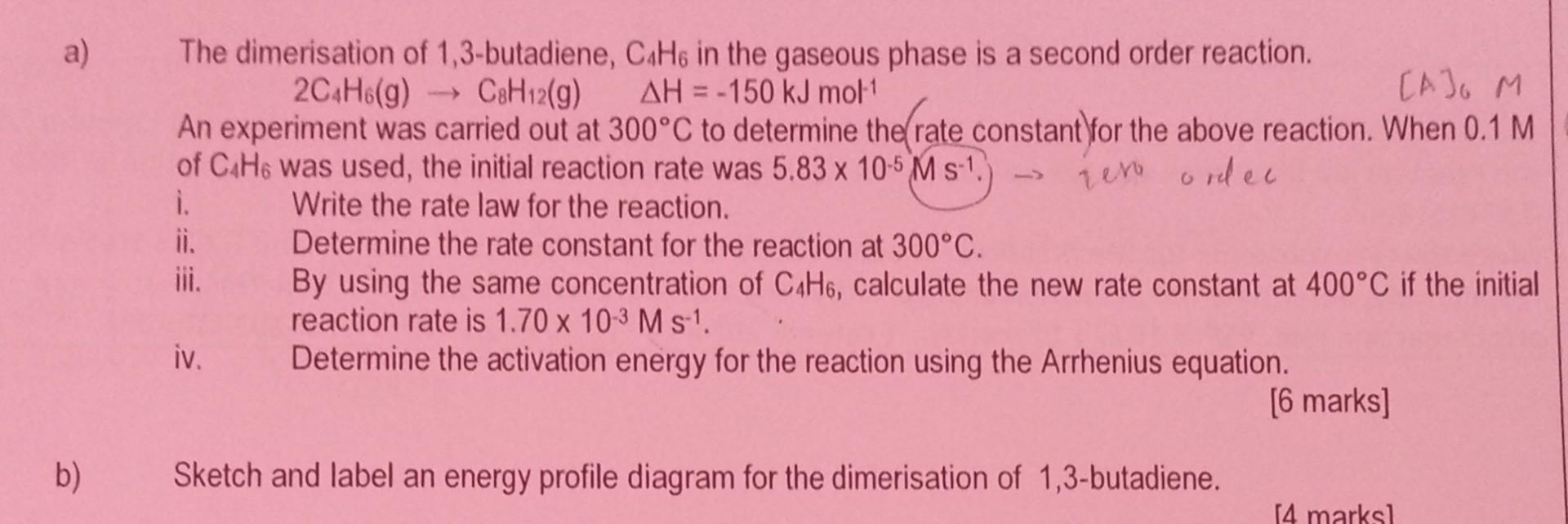
a) The dimerisation of 1,3-butadiene, C4H6 in the gaseous phase is a second order reaction. 2C4H6(g)C8H12(g)H=150kJmol1 [A]6M An experiment was carried out at 300C to determine the (rate constant)for the above reaction. When 0.1M of C4H6 was used, the initial reaction rate was 5.83105Ms1.) ievo ordec i. Write the rate law for the reaction. ii. Determine the rate constant for the reaction at 300C. iii. By using the same concentration of C4H6, calculate the new rate constant at 400C if the initial reaction rate is 1.70103Ms1. iv. Determine the activation energy for the reaction using the Arrhenius equation. [6 marks] b) Sketch and label an energy profile diagram for the dimerisation of 1,3-butadiene
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


