Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) The vapour pressure of water at 90C is 70.1 kPa and the mean latent heat of vaporization between 90 and 98C may be taken
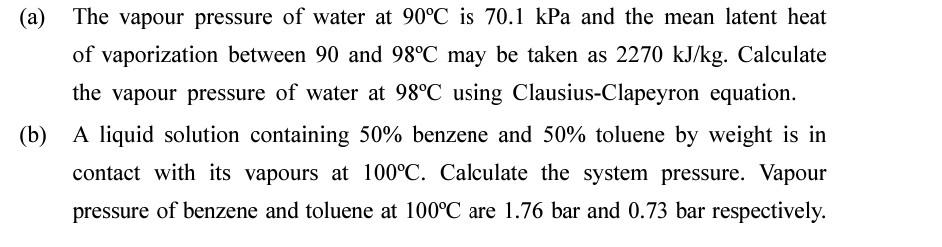
(a) The vapour pressure of water at 90C is 70.1 kPa and the mean latent heat of vaporization between 90 and 98C may be taken as 2270 kJ/kg. Calculate the vapour pressure of water at 98C using Clausius-Clapeyron equation. (b) A liquid solution containing 50% benzene and 50% toluene by weight is in contact with its vapours at 100C. Calculate the system pressure. Vapour pressure of benzene and toluene at 100C are 1.76 bar and 0.73 bar respectively
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


